Abstract
Breast cancer metastases to the gastrointestinal tract are very rare occurrences. Among the histological subtypes of breast cancer, invasive lobular carcinomas have a high capacity of metastasis to uncommon sites including the stomach. Conversely, there has not been sufficient evidence supporting the gastric metastasis of invasive ductal carcinoma. Herein, we report a unique case of metastatic ductal breast carcinoma mimicking primary linitis plastica in a male patient, particularly focusing on the clinical and pathological features of presentation. Moreover, we propose a immunohistochemical panel of selected antibodies including those for cytokeratin 20, cytokeratin 7, estrogen receptor, progesterone receptor, E-cadherin, gross cystic disease fluid protein 15, and GATA binding protein 3 for an accurate differential diagnosis.
Breast cancer is the most common malignancy after lung cancer and the leading cause of cancer-related deaths among women worldwide [1]. Although the incidence of breast cancer has been increasing, male breast cancer is a rare and uncommon disease, accounting for only 0.5% to 1% of all cases. Male patients are diagnosed at late stages and usually have a worse prognosis, mainly due to the lack of screening for disease prevention and less awareness of pathognomonic symptoms [2]. Nonetheless, there have been several studies where male sex was not reported as an independent prognostic factor for worse clinical outcome and there were no meaningful differences between the 5- and 15-year excess mortalities of men and women [2]. Among the histological subtypes of breast cancer, invasive lobular carcinomas have a high capacity to metastasize to uncommon sites such as the skin, pleura, peritoneum, ovaries, and the gastrointestinal (GI) tract [3]. In such cases, the stomach is the most affected segment of the digestive tract, detected in 3% to 18% of patients with metastatic disease. On the other hand, the metastases of invasive ductal carcinomas to the GI tract are rare. Importantly, metastatic spread to the stomach may occur many years after the initial treatment for breast cancer, and it may be extremely difficult to distinguish from a primary gastric cancer based on clinical, endoscopic, radiological, and histopathological features. However, this discrimination is imperative to establish the best treatment strategy for our patients. Herein, we report the first case of ductal breast carcinoma metastatic to the stomach mimicking primary linitis plastica in a male patient.
A 65-year-old man presented to the emergency room of Santa Maria della Misericordia Hospital (Perugia, Italy) with the primary complaints of hematemesis and epigastric pain in April 2015. Twelve years ago, he had undergone total mastectomy with complete axillary dissection for invasive ductal carcinoma. Postoperative histology showed a completely excised invasive ductal carcinoma with the following immunohistochemistry (IHC) profile: estrogen receptor (ER), 90%; progesterone receptor (PR), 0%; Ki-67, >10%; and human epidermal growth factor receptor 2 (HER2), 1+ (Figure 1). The patient was treated with four cycles of adjuvant doxorubicin and cyclophosphamide, followed by 5 years of tamoxifen with no signs of recurrence. In August 2011, the relapse was observed at the surgical mastectomy site and the patient underwent radical surgical excision of the nodule, which was diagnosed as metastasis from invasive ductal breast carcinoma with the same IHC profile as the primary tumor (ER, 100%; PR, 0%; Ki-67, 14%; HER2, 1+). Following these findings, the patient was treated with 12 cycles of doxorubicin, cyclophosphamide, and paclitaxel, followed by tamoxifen with no signs of recurrence until September 2014, when a positron emission tomography/computed tomography (PET/CT) image showed new bone lesions. Subsequently, tamoxifen treatment was stopped and the patient was started on anastrozole; he showed stable disease on the subsequent follow-up visits and instrumental evaluations.
At the time of hospitalization in April 2015, the patient had undergone an esophagogastroduodenoscopy, which had revealed a Borrmann type 4 tumor that was characterized by diffuse thickening and sclerosis of the gastric wall and marked hypertrophy of the mucosal folds. The biopsy specimens of the stomach demonstrated poorly differentiated diffuse adenocarcinoma. A subsequent PET/CT scan showed wall thickening of both the gastric antrum and the body of the stomach with a significant 18F-fluorodeoxyglucose (FDG) uptake, indicating linitis plastica (Figure 2). Based on these findings, the patient was diagnosed with primary gastric adenocarcinoma, although the possibility of a metastatic progression of invasive ductal carcinoma to the stomach could not be eliminated. However, considering the poorer prognosis associated with primary diffuse gastric carcinoma compared to oligometastatic breast cancer and the different treatment strategies associated with these diagnoses, after a multidisciplinary discussion, it was decided that surgery was the most suitable treatment strategy. Therefore, the patient underwent gastrectomy with Roux-en-Y esophagojejunal anastomosis with both diagnostic and therapeutic intention.
On macroscopic examination, the stomach measured 163 mm with wall thickening (up to 10 mm) of the antrum and corpus. The internal surface of the thickened wall showed a diffuse loss of mucosal rugae in an 88-mm region without mucosal ulceration. The whole area was sampled with serial sections. Microscopic examination revealed diffuse growth of a poorly differentiated carcinoma with a full-thickness extension through the stomach wall. The neoplasm appeared to grow from the serous surface toward the overlying gastric mucosa, which appeared normal but infiltrated. Foci of dysplasia were not found in the surrounding gastric mucosa, leading to the suspicion that the gastric involvement was of metastatic origin. The tumor cells had large vesicular nuclei with prominent nucleoli, and they were arranged in solid and tubular patterns of growth (Figure 3A, B). The mitotic count was elevated (up to 20 mitosis/10 high power fields). In addition, some aspects of perineural and lymphovascular invasion were present. Importantly, the tumor cells showed immunore-activity for cytokeratin 7 (CK7), E-cadherin, gross cystic disease fluid protein 15 (GCDFP15), and GATA binding protein 3 (GATA3), consistent with the findings for metastatic breast carcinoma of no special type (Figure 3C-E). The ER, PR, CK20, and synaptophysin statuses were negative, whereas IHC staining for HER2 revealed a 3+ positivity (Figure 3F). A PET/CT scan performed after the gastric resection showed no FDG uptake (Figure 2G, H). Following diagnosis of HER2 positive gastric metastasis of breast carcinoma, the patient received six cycles of docetaxel plus trastuzumab, thus achieving stable disease. Presently, the patient is receiving maintenance therapy with trastuzumab, without any signs of progression.
Breast cancer metastases to the GI tract are very rare occurrences; the most common metastatic sites are the bones, liver, lungs, and brain [4]. The estimated overall incidence of breast cancer metastasis to the stomach during either long-term follow-up or post mortem analyses is 2% to 18%, with 90% to 94% of the patients having concurrent breast cancer metastases [34]. Importantly, metastases to the GI tract can represent the first manifestation of metastatic breast cancer, as well as late recurrence even years after the diagnosis of the primary neoplasm, similar to the present case. Of note, the potential of metastasis to the stomach is higher among patients with lobular histologies. In this respect, a retrospective analysis conducted over a period of 15 years demonstrated that 83% of patients with gastric metastases had lobular histologies [4]. These findings were confirmed in a larger series, which demonstrated that the lobular type accounted for at least 83% to 85% of the cases with gastric metastases form breast cancer [56]. The biological mechanism underlying the unusual spread of lobular breast carcinoma to the GI tract is not clearly understood; however, some authors have hypothesized that it might be linked to a specific tropism of the lobular cells [678]. Intriguingly, in the mixed histology subtype (ductal and lobular), only the lobular component potentially metastasizes to the stomach [34]. Clinically, metastatic gastric linitis plastica is indistinguishable from a primary gastric linitis plastica, similar to the present case. In fact, the clinical presentation of gastric involvement is completely a specific; it commonly manifests as epigastric pain, indigestion, lack of appetite, and occasional gastric obstruction and bleeding [78]. Although rare, linitis plastica represents the most frequent type of gastric metastasis from lobular breast cancer [789]. Conversely, the evidence for the association of ductal breast carcinoma with gastric metastases is insufficient, and its association with linitis plastica is very rare as it usually manifests as a polyp, ulcer erosion, or stenosis [789]. In the present case, the patient showed tumor recurrence in the GI tract, presenting as secondary linitis plastica from a primary breast ductal carcinoma, as the second case reported in medical literature [10], and the first ever described case among male patients.
In order to obtain an accurate differential diagnosis between breast cancer metastasis to the stomach and primary gastric cancer, IHC analysis is strongly recommended, because clinical, radiological, and endoscopic findings are generally unhelpful. Importantly, even though the detection of ER and PR expression in biopsy samples and surgical gastric specimens is indicative of breast cancer metastasis to the stomach, early studies with first-generation antibodies documented ER and PR positivity in 32% and 12% of the cases, respectively, in patients with primary gastric cancer [11]. Later, van Velthuysen et al. [12] demonstrated that second-generation anti-ER antibodies are sensitive and specific biomarkers for determining breast origin, because they are not usually expressed by gastric cancer cells. Unfortunately, they are unhelpful in the case of ER-negative primary breast cancer, and loss of ER expression in the metastatic site of breast cancer has been widely reported, as observed in our patient. Therefore, in such cases, ER and PR do not represent suitable biomarkers to discriminate between breast cancer metastasis to the stomach and primary gastric cancer. In the present study, the authors provide an IHC panel of selected antibodies that allowed a proper differential diagnosis (CK20, CK7, ER, PR, E-cadherin, GCDFP15, and GATA3).
GCDFP15 has been proven an accurate biomarker for identifying a malignant lesion of breast origin, yielding 55% to 76% sensitivity, and 95% to 100% specificity. Additionally, combined IHC for CK7 and CK20 represents another useful tool, as breast carcinomas are CK7 positive in 90% of cases versus the 50% to 55% of primary gastric cancers, whereas CK20 is negative in all breast carcinomas and highly positive in gastric, colorectal, and pancreatic carcinomas [13]. Notably, our panel also included GATA3, a member of the GATA family of zinc-finger DNA binding proteins, which is currently considered a reliable, sensitive, and specific immunomarker for the diagnosis of breast cancer, as it was found only in breast and urothelial carcinomas but not in other tumors [14]. Lastly, the E-cadherin expression was evaluated. Aberrant E-cadherin expression is common in invasive ductal carcinomas that develop distant metastases [1214]. Intriguingly, distant metastases consistently express E-cadherin, often more strongly than the primary tumor [14]. When breast cancer metastasis to the GI tract is suspected, positive IHC for CK7, GCDFP15, and GATA3 can effectively confirm the diagnosis, particularly in case of CK20 negativity. In addition, E-cadherin expression further corroborates a ductal histology, whereas E-cadherin loss is peculiar of invasive lobular carcinomas. In the present case, discordance was observed between the hormone receptor statuses of primary (ER, 90%; PR, 0%) and metastatic breast cancer (ER, 0%; PR, 0%). Loss of ER and PR during disease progression is a well-recognized phenomenon, representing a consequence of disease progression or prolonged endocrine therapy [1213]. Concerning the HER2 status, we reported discordance between primary invasive ductal carcinoma and gastric recurrence as well. As opposed to the primary tumor, the immunohistochemical staining for HER2 on gastric specimens revealed 3+ positivity that supported targeted therapy with trastuzumab, which in turn resulted in stable disease. Data on treatment are extremely limited because of the lack of randomized trials and the lack of reports; however, majority of the patients with gastric metastases from breast cancer usually receive chemotherapy or hormone therapy, based on the assessment of hormone receptor statuses in metastatic tissues. In case of isolated GI tract involvement, surgery should be considered where appropriate, although it is often reserved for palliation purposes in cases of intestinal obstruction or bleeding. Conversely, in the multi-metastatic setting, surgery does not improve survival compared to systemic treatment. Of note, to the best of our knowledge, trastuzumab-based therapy for HER2 positive gastric metastasis from breast cancer has never been reported previously. Beyond the rarity of the case and its presentation, this report further enlarges the growing body of evidence suggesting that hormone receptor and HER2 status should be determined for all recurrences, in case the procedure is technically feasible and not unreasonably invasive, since any change in ER, PR, and HER2 statuses might grant new therapeutic possibilities for the patient, allowing us to tailor the treatment in the most accurate manner.
Figures and Tables
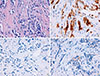 | Figure 1Pathologic findings of primary breast carcinoma. (A) Microphotograph showing invasive ductal carcinoma of the breast growing within a desmoplastic stroma (H&E stain, ×400). (B) Immunohistochemical stain showing positivity for estrogen receptor (90%) (×400). (C) Immunohistochemical stain showing absence of expression of progesterone receptor (×400). (D) Immunohistochemical stain showing negativity for human epidermal growth factor receptor 2 expression (1+) (×400). The aforementioned histologic features and the immunohistochemical expression pattern of the tumor were consistent with a diagnosis of invasive breast ductal carcinoma. |
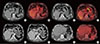 | Figure 2Pre- and postoperative positron emission tomography/computed tomography (PET/CT) finding. (A) Unenhanced CT and (B) Fusion PET/CT axial images showing no gastric fluorodeoxyglucose (FDG) uptake neither gastric wall thickening. (C, D) PET/CT showing the wall thickening of the gastric antrum (white arrow head) with a significant FDG uptake (black arrow head). (E) CT axial arterial and (F) venous phase, respectively at 35 and 80 seconds after intravenous iodinate contrast medium injection, showing the progression of the wall thickening, now involving the antrum and the body of the stomach, figuring out a linitis plastic (white arrow heads). (G, H) PET/CT axial images performed after the gastric resection showing no FDG uptake at the level of Roux-en-Y esophagojejunal anastomosis. |
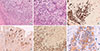 | Figure 3Pathologic findings of gastric recurrence. (A) microphotograph showing the stomach wall infiltrated by the neoplasm, arranged in tubular and solid pattern of growth (H&E stain, ×100). (B) Note the infiltration of tumor cells in the normal gastric mucosa, which have large vesicular nuclei with prominent nucleoli (H&E stain, ×200). (C) Immunohistochemical stain showing immunoreactivity for cytokeratin 7 (×100). (D) Immunohistochemical stain showing immunoreactivity for gross cystic disease fluid protein 15 (×100). (E) Immunohistochemical stain showing immunoreactivity for GATA binding protein 3 (GATA3) (×100). (F) Immunohistochemical stain showing positivity for human epidermal growth factor receptor 2 expression (×400). In the lacking of immunohistochemistry positivity to hormone receptors, the combined use of gross cystic disease fluid protein 15, GATA3 and cytokeratin 7 allowed for correct diagnosis of metastatic ductal breast cancer to the stomach. |
References
2. Deb S, Lakhani SR, Ottini L, Fox SB. The cancer genetics and pathology of male breast cancer. Histopathology. 2016; 68:110–118.

3. Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014; 232:23–31.

4. Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: I. stomach. Gastrointest Endosc. 1992; 38:130–135.

5. Ferri LE, Onerheim R, Emond C. Linitis plastica as the first indication of metastatic lobular carcinoma of the breast: case report and literature review. Can J Surg. 1999; 42:466–469.
6. Ciulla A, Castronovo G, Tomasello G, Maiorana AM, Russo L, Daniele E, et al. Gastric metastases originating from occult breast lobular carcinoma: diagnostic and therapeutic problems. World J Surg Oncol. 2008; 6:78.

7. Madeya S, Börsch G. Gastrointestinal metastases of breast carcinoma. Gastrointest Endosc. 1993; 39:103–104.

8. Almubarak MM, Laé M, Cacheux W, de Cremoux P, Pierga JY, Reyal F, et al. Gastric metastasis of breast cancer: a single centre retrospective study. Dig Liver Dis. 2011; 43:823–827.

9. Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol. 2006; 12:6219–6224.

10. Yagi Y, Sasaki S, Yoshikawa A, Tsukioka Y, Fukushima W, Fujimura T, et al. Metastatic gastric carcinoma from breast cancer mimicking primary linitis plastica: a case report. Oncol Lett. 2015; 10:3483–3487.

11. Matsui M, Kojima O, Kawakami S, Uehara Y, Takahashi T. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992; 22:421–425.

12. van Velthuysen ML, Taal BG, van der Hoeven JJ, Peterse JL. Expression of oestrogen receptor and loss of E-cadherin are diagnostic for gastric metastasis of breast carcinoma. Histopathology. 2005; 46:153–157.

13. O'Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med. 2005; 129:338–347.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download