Abstract
Purpose
We aimed to compare the detection of breast cancer using full-field digital mammography (FFDM), FFDM with computer-aided detection (FFDM+CAD), ultrasound (US), and FFDM+CAD plus US (FFDM+CAD+US), and to investigate the factors affecting cancer detection.
Methods
In this retrospective study conducted from 2008 to 2012, 48,251 women underwent FFDM and US for cancer screening. One hundred seventy-one breast cancers were detected: 115 invasive cancers and 56 carcinomas in situ. Two radiologists evaluated the imaging findings of FFDM, FFDM+CAD, and US, based on the Breast Imaging Reporting and Data System lexicon of the American College of Radiology by consensus. We reviewed the clinical and the pathological data to investigate factors affecting cancer detection. We statistically used generalized estimation equations with a logit link to compare the cancer detectability of different imaging modalities. To compare the various factors affecting detection versus nondetection, we used Wilcoxon rank sum, chi-square, or Fisher exact test.
Results
The detectability of breast cancer by US (96.5%) or FFDM+CAD+US (100%) was superior to that of FFDM (87.1%) (p=0.019 or p<0.001, respectively) or FFDM+ CAD (88.3%) (p=0.050 or p<0.001, respectively). However, cancer detectability was not significantly different between FFDM versus FFDM+CAD (p=1.000) and US alone versus FFDM+CAD+US (p=0.126). The tumor size influenced cancer detectability by all imaging modalities (p<0.050). In FFDM and FFDM+CAD, the nondetecting group consisted of younger patients and patients with a denser breast composition (p<0.050). In breast US, carcinoma in situ was more frequent in the nondetecting group (p=0.014).
Mammography is the only recommended imaging tool for breast cancer screening by the guidelines of the World Health Organization and the Korea National Cancer Screening Program. Full-field digital mammography (FFDM) offers many advantages such as higher contrast resolution, better dynamic range, and lower noise, compared to conventional film-screen mammography (FSM) [12]. The Digital Mammographic Imaging Screening Trial (DMIST) has demonstrated that the diagnostic accuracy of FFDM is significantly higher than that of FSM in women younger than 50 years and in women with dense breasts on mammography [34]. The DMIST results show an improvement of 55% to 70% in cancer detection, compared to FFDM with FSM; however, a substantial number of cancers cannot be detected, even with FFDM. Therefore, supplementary imaging tools to improve the detectability of breast cancer are needed.
A computer-aided detection (CAD) system is a software program for the automatic analysis of mammography. The application of CAD to FFDM is helpful in reducing false-negative interpretations in screening populations [567]. Warren Burhenne et al. [6] reported that CAD correctly marked undetected findings in 77.4% (89/115) of prior false-negative mammograms. Dean and Ilvento [7] found that CAD detected more cancers in screening patients and in diagnostic patients with no deterioration in positive predictive values.
Breast ultrasound (US) is widely used as a supplementary imaging modality for evaluating mammographically detected abnormalities, and is an effective screening tool for detecting occult breast cancers in mammographically dense breasts [891011]. US screening offers effective detection of occult small breast cancers [9]; its cancer detection rate is similar to or higher than that of screening mammography (0.30%–0.46%) [891011].
Most Asian women, including Korean women, have a dense breast composition; thus, screening mammography is limited in its ability to detect small breast cancer lesions. In addition, mammography examination produces radiation exposure. Evaluating the diagnostic performances of mammography and additional imaging modalities to find out which combination yields better diagnostic performances yet with less harm to the patient is necessary to modify screening programs. To date, no report has compared the breast cancer detection rates between FFDM with the application of CAD (FFDM+CAD) and breast US in a screening population. The purpose of this study was to compare breast cancer detection rates between FFDM, FFDM+CAD, breast US, and FFDM+CAD plus US (FFDM+CAD+US), and to investigate any radiological and clinicopathological factors that affect breast cancer detection.
This retrospective study was approved by the Institutional Review Board (IRB approval numbers: AN15299-001 and AS15197-002) of Korea University Anam Hospital and Korea University Ansan Hospital and did not require informed consent. From January 2008 to December 2012, 48,251 women underwent FFDM and US for breast cancer screening in our institution. Of these, we searched the database for screened and detected breast cancer lesions in patients for whom both FFDM and breast US had been performed, and 171 breast cancer lesions were detected. A retrospective study was conducted using these 171 cancerous lesions. The patients' cancer types were 115 (67.3%) invasive carcinomas and 56 (32.7%) ductal carcinomas in situ (DCIS). The pathologic subtypes of the invasive carcinomas were invasive ductal carcinoma (n=98), invasive lobular carcinoma (n=3), microinvasive DCIS (n=7), and others (n=7; papillary, mucinous, meta-plastic, tubular, and medullary carcinomas).
Two Selenia FFDM units (Hologic, Denver, USA) were used. Mediolateral oblique and craniocaudal projections of both breasts were obtained for each patient. As the CAD for FFDM, we used R2 Image Checker, version 8.3 (R2 Technology Inc., Sunnyvale, USA). It marks suspected mass lesions with asterisks (*), calcifications with triangles (▲), and masses with calcifications with crosses (+). The US examination was performed using the iU22 system (Philips Medical Systems, Bothell, USA) or Logiq 9 unit (General Electric, Milwaukee, USA) with a high-frequency broadband width linear array transducer. The US examination was performed in the bilateral whole breasts and axillae, and survey scanning was performed in the transverse and the sagittal planes.
Two radiologists (S.E.S. and K.R.C.) with 3 and 12 years of experience, respectively, in breast imaging interpreted all images using a picture archiving and communication system. One radiologist had 3 years of experience in CAD reading and the other radiologist had 7 years of experience. For the evaluation of FFDM and CAD images, we used the Coronis 5MP display system (Barco, Duluth, USA) with two 5-million-pixel gray-scale Liquid Crystal Display monitors. To ensure that images were displayed with the highest possible fidelity, the display system was calibrated with a dual-head BarcoMed 5MP2FH display controller (Barco) and MediCal Pro software (Barco). All digital images had the window width and level settings adjusted to optimize the image display. To evaluate the US images, we used the Star PACS system (Infinitt, Seoul, Korea). We used consensus double reading. Each reader independently evaluated the images, and the final decision was reached by discussion between the two readers. At a 3-week interval so as not to memorize previous images, the readers independently reviewed the imaging findings of FFDM, FFDM+CAD, and breast US. The readers were blinded to the other imaging findings, the clinical data, and the pathological results.
We evaluated mammography and breast US, based on the Breast Imaging Reporting and Data System (BI-RADS) lexicon of the American College of Radiology [12]. On FFDM, we evaluated breast composition and abnormal findings. The breast composition was classified into four types: 1, almost entirely fatty; 2, scattered area of fibroglandular density; 3, heterogeneously dense breast; and 4, extremely dense breast. The abnormal findings on mammography were classified as a mass, calcification, a mass with calcification, asymmetry, or architectural distortion. The US findings were classified as a mass, a ductal change, a mass with calcification, or a ductal change with calcification. After we evaluated the mammography or US images independently at a 3-week interval, we assessed the BI-RADS category for each imaging modality. We followed the definition of positive screening examination in the follow-up and outcome monitoring of the BI-RADS lexicon. [12]. The positive screening examination is defined as BI-RADS category 0, 3, 4, or 5, based on the lexicon. On FFDM or breast US, we considered a result as "positive" if there was a breast lesion with a BI-RADS category 0, 3, 4, or 5 on each imaging modality. We determined FFDM+CAD as "positive" when the CAD system marked the exact lesion site on craniocaudal and/or mediolateral oblique mammography. We regarded FFDM+CAD+US detection as a "positive" if there was a positive examination on FFDM, CAD, or breast US.
We also reviewed the clinical and pathological findings of all patients to search for factors that affected cancer detection in the different breast imaging modalities (i.e., FFDM, FFDM+CAD, or US). We evaluated patient age, pathological cancer size and type (i.e., invasive carcinoma or DCIS), and breast composition on mammography as the affecting factors. The patients were divided into two groups (i.e., "detecting" and "nondetecting") for each imaging modality, and we evaluated any difference in various factors between these two groups.
A statistician (J.C.) performed statistical analysis by using SPSS version 11.0 (SPSS Inc., Chicago, USA) and SAS version 9.2 (SAS Institute Inc., Cary, USA), and a p-value less than 0.05 was statistically significant. The generalized estimation equations with a logit link [13] was applied to compare the cancer detect-ability of the imaging modalities. In addition, a Bonferroni correction method was used for multiple comparisons. To compare the affecting factors between the detecting and non-detecting groups in each imaging modality, we conducted the Wilcoxon rank sum test to analyze continuous variables because they did not satisfy the normality assumption. We used the chi-square or the Fisher exact test for the categorical variables.
On pathologic examination, the tumor types were 115 invasive carcinomas (67.3%) and 56 DCIS (32.7%). The tumor sizes ranged 2 to 42 mm (median, cancer=15 mm, non-cancer=8 mm). Of the 171 cancerous lesions, 125 lesions (73.1%) were less than 20 mm. Table 1 demonstrates the radiological findings of the breast cancers on FFDM and breast US. On FFDM, cancers were depicted as a mass (n=32), calcification (n=43), mass with calcification (n=48), focal asymmetries (n=24) (Figure 1), or architectural distortions (n=2). The remaining 22 cancerous lesions (12.8%) were not detected on FFDM (Figure 2). There were 12 invasive carcinomas and 10 DCIS. Twenty (90.9%) of the undetected 22 cancers on FFDM were detected as masses (n=17) or ductal changes (n=3) on breast US. Two cancerous lesions among the 22 undetected on FFDM were detected when CAD was added to FFDM: one invasive carcinoma and one DCIS. The CAD marked triangles on these two lesions, indicating calcifications.
On breast US, cancers were found as a mass (n=93) (Figure 2), a ductal change (n=14) (Figure 1), a mass with calcifications (n=42), or a ductal change with calcifications (n=16) (Table 1). Among the 43 lesions that manifested as calcifications only on FFDM or FFDM+CAD, 39 (90.7%) were detected on US as a mass with calcifications (n=20), a ductal change with calcifications (n=11), a ductal change (n=4), or a mass (n=4). The remaining six lesions (3.5%) were not detected using US alone. The undetected cancerous lesions on breast US were one invasive carcinoma and five DCIS. All of these undetected lesions were detected as calcifications on FFDM+CAD, and four of these lesions were detected using FFDM alone.
Table 2 shows the detectability of breast cancer lesions by the various imaging modalities. The detectability was 87.1% (149/171) by FFDM, 88.3% (151/171) by FFDM+CAD, 96.5% (165/171) by US screening alone, and 100% (171/171) by FFDM+CAD+US. These overall differences in cancer detect-ability were statistically significant (p<0.001). In addition, the detectability between the various imaging modalities was also different for the pathological tumor types and for patients with invasive carcinoma or DCIS (p<0.001).
Table 3 presents the p-values of the multiple comparisons of cancer detectability for the various imaging modalities. The overall detectability of US screening alone (96.5%) was superior to that of FFDM (87.1%) and FFDM+CAD (88.3%) (p=0.019 and p=0.050, respectively). The detectability of US screening (99.1%) for patients with invasive carcinoma was also significantly higher than that of FFDM (89.6%) or FFDM+CAD (90.4%) (p=0.015 and p=0.027, respectively). We also found that FFDM+CAD+US was superior to FFDM or FFDM+CAD in patients with invasive carcinoma (100% vs. 89.6% or 100% vs. 90.4%) (p=0.003 or p=0.005, respectively) and in patients with DCIS (100% vs. 82.1% or 100% vs. 83.9%) (p=0.005 or p=0.011, respectively). However, cancer detectability was not significantly different between FFDM vs. FFDM+CAD (p=1.000) and screening US alone vs. FFDM+CAD+US (p=0.126).
Table 4 shows the detectability of breast cancer in FFDM and FFDM+CAD, based on breast composition. On mammography, breast composition was type 1 in 12 patients (7.0%), type 2 in 50 patients (29.2%), type 3 in 95 patients (55.6%), and type 4 in 14 patients (8.2%). The detectability of FFDM and FFDM+CAD was decreased in dense breast tissues and this decrease was statistically significant (p=0.039 and p=0.013, respectively). When we used CAD, two cancers (one cancer with type 2 breast composition and one cancer with type 3 breast composition) were detected as calcifications.
Table 5 presents the factors affecting the breast cancer detectability of each imaging modality. We compared the clinical, radiological, and pathological factors between the detecting and the nondetecting groups. The tumor size affected cancer detectability in all imaging modalities with the nondetecting group having smaller tumor sizes, compared to the detecting group (p<0.050). In FFDM and FFDM+CAD, age and breast composition affected cancer detectability (p<0.050). The nondetecting group comprised patients with a young age and dense breast composition. However, the pathologic tumor type, whether invasive carcinoma or DCIS, did not affect tumor detection in FFDM and FFDM+CAD (p=0.173 and p=0.214, respectively). On the other hand, the pathologic tumor type did affect cancer detection in breast US (p=0.014). Five of the six (83.3%) nondetecting cases in US were DCIS.
Mammography has been used as a screening tool in breast cancer for the past 30 years. Randomized controlled trials of mammographic screening for breast cancer demonstrate that screening reduces disease mortality by 20% to 30% [1415]. However, a substantial number of cancerous lesions are undetected on mammography, and the mammographic sensitivity substantially decreases with a sensitivity as low as 30% to 48% in women with dense breasts [16]. To address this limitation, applying CAD to mammography or breast US has been an adjunctive tool.
In previous studies, the sensitivity of FFDM+CAD varied from 78% to 96% [1718192021]. On mammography, the sensitivity of FFDM+CAD can be influenced by the lesion type. CAD classifies breast lesions into three types: a mass, a mass with calcification, or calcification only; it does not differentiate masses from asymmetries or architectural distortions, although CAD does show greater sensitivity for calcifications than for mass lesions such as masses, architectural distortions, or asymmetries [1720212223]. Indistinct or subtle mass lesions cannot be detected in the CAD system because of the low contrast between lesions and the background [21]. In the current study, the detectability of breast cancer in FFDM+CAD was 88.3%. When compared with the detectability of FFDM (87.1%), there was no significant difference in overall cancers, invasive cancers, or DCIS. Two unfound cancerous lesions in FFDM were correctly detected after CAD application. These were a few microcalcifications with grouped distribution in extremely dense breasts. Breast US also did not find these lesions; thus, only CAD was able to detect these two lesions with grouped calcifications.
Based on our results, breast cancer detection in FFDM and FFDM+CAD was affected by patient age, tumor size, and breast composition, with patient age and breast composition correlated with breast density on mammography. Mandelson et al. [16] determined that breast density is one of the strongest predictors of the failure of mammographic screening in detecting cancer. Breast density is a risk factor for undetected cancerous lesions, and false-positive and false-negative mammographic interpretations are more likely with dense breasts. The CAD application cannot overcome the fundamental limitation of mammography, which is related to breast density. A previous study by Kim et al. [24] found that the frequency of dense breasts was much higher among Korean women in their forties than among Western women; therefore, the detectability of breast cancer in Korean women was lower than that of Western women in their forties. In addition, the forties represent the peak incidence of breast cancer in Korea; thus, additional or substitutional imaging methods are necessary to improve breast cancer detection in dense breasts.
The use of breast US in women with dense breasts is promising. Korpraphong et al. [22] reported that use of US as an adjunct to mammography showed a significant benefit for the detection of breast cancer in asymptomatic average-risk women with nonfatty breasts. The cancer detection rate when using additional US was 1.4 per 1,000 examinations; there was more significant improvement in women aged 40 to 59 years and in women with extreme parenchymal density. In our study, breast US was more sensitive for cancer detection than FFDM or FFDM+CAD for overall cancers or invasive cancers. In addition, there was no significant difference in cancer detectability between US alone vs. FFDM+CAD+US. Breast US detected 90.9% (20/22) of the undetected cancers on FFDM. These presented as masses or ductal changes on breast US.
The development of computer system and image processing techniques of US has improved the detection and visualization of calcifications in the breast. In the current study, 90.7%(39/43) of cancers with calcifications only on FFDM or FFDM+CAD were detected on US as a mass with calcification, a ductal change with calcification, a ductal change, or a mass. According to Kim et al. [25], because of increased specificity, breast US improves the diagnostic performance with regard to grouped calcifications without associated masses on mammography. On breast US, malignant calcifications are more commonly associated with a mass or a ductal change, and thus can be more easily detected rather than benign calcifications. To date, however, US is limited with regard to a small cluster of calcifications. In this study, all six of the undetected cancerous lesions on US were small grouped calcifications that ranged 3 to14 mm (mean size, 10.50 mm); five of these six lesions were DCIS. Two of these six undetected cancers on US were also not detected on FFDM. In breast DCIS, mammography cannot detect 6% to 23% of DCIS lesions, especially in dense breasts [2627]. There are a few reports about the role of breast US in DCIS [2728]. According to Jin et al. [28], US can reveal occult DCIS in patients with dense breasts and have an important role in detecting DCIS and in evaluating pathologic features. The diagnostic accuracy in the detection of DCIS on breast US is associated with a higher grade, microinvasion, or comedonecrosis. In addition, most DCISs detected by US alone are localized lesions with few extensive intraductal component and are of low grade, which suggests a successful local excision [29]. Based on our results, breast cancer detection on breast US was not influenced by patient age and breast density. Therefore, US can be recommended as a screening tool for all age groups.
This study has several limitations. First, this was a retrospective study, and thus we could not evaluate the sole effect of screening US on the clinicians' daily workflow. US examination was mostly performed after FFDM scanning. We reviewed FFDM, FFDM+CAD, or breast US images independently, were blind to each other, and reviewed the images at a 3-week interval so as not to memorize previous images; however, the second review of the images may be different with the initial perception of breast lesions during US examination. In addition, because of the retrospective study design, we were unable to assess the effect of CAD on workflow or examine the reproducibility of CAD results. Second, we included true-positive cancers in this study, thus, we could not obtain false-positive rate for cancer screening. Third, we did not calculate the interobserver variability for image assessment because two radiologists evaluated breast images by consensus in the current study.
In conclusion, US examination for breast cancer screening is satisfactory for all age groups and breast compositions. Applying CAD to FFDM does not have an additional role in cancer screening. However, the detectability of breast US is influenced by tumor type and size; in particular, DCIS with small grouped calcifications cannot be detected. Therefore, we would recommend further studies on effective breast screening protocols with US.
Figures and Tables
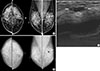 | Figure 1Mammographic and ultrasound findings of a 35-year-old woman with ductal carcinomas in situ (DCIS). Screening mammography (A) demonstrated a focal asymmetry in the left upper breast (arrow) and computer-aided detection applied mammography (B) detected the lesion (asterisk, marked by computer-aided detection program) (arrow). The breast ultrasound (C) demonstrated a ductal change in the left upper breast (arrows); this was a pathologically proven DCIS. |
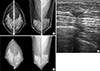 | Figure 2Mammographic and ultrasound findings of a 46-year-old woman with invasive ductal carcinoma. Screening mammography (A) and computer-aided detection applied mammography (B) did not show any abnormal findings. The breast ultrasound (C) demonstrated an indistinct oval hypoechoic mass in the left upper outer quadrant (arrows); this was a pathologically verified cancer. |
Table 1
Radiological findings of 171 breast cancers

Table 2
The detectability of breast cancer using various imaging modalities

Table 3
The p-values for multiple comparison of cancer detectability using various imaging modalities
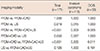
Table 4
The detectability of breast cancer in FFDM or FFDM+CAD according to breast composition

| Breast composition | FFDM, No. (%) | FFDM+CAD, No. (%) |
|---|---|---|
| 1 | 11/12 (91.7) | 11/12 (91.7) |
| 2 | 48/50 (96.0) | 49/50 (98.0) |
| 3 | 80/95 (84.2) | 81/95 (85.3) |
| 4 | 10/14 (71.4) | 10/14 (71.4) |
| p-value | 0.039 | 0.013 |
Table 5
Factors affecting breast cancer detectability

Notes
References
2. Shtern F. Digital mammography and related technologies: a perspective from the National Cancer Institute. Radiology. 1992; 183:629–630.

3. Pisano ED, Gatsonis CA, Yaffe MJ, Hendrick RE, Tosteson AN, Fryback DG, et al. American College of Radiology Imaging Network digital mammographic imaging screening trial: objectives and methodology. Radiology. 2005; 236:404–412.

4. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005; 353:1773–1783.

5. Brem RF, Baum J, Lechner M, Kaplan S, Souders S, Naul LG, et al. Improvement in sensitivity of screening mammography with computer-aided detection: a multiinstitutional trial. AJR Am J Roentgenol. 2003; 181:687–693.

6. Warren Burhenne LJ, Wood SA, D'Orsi CJ, Feig SA, Kopans DB, O'Shaughnessy KF, et al. Potential contribution of computer-aided detection to the sensitivity of screening mammography. Radiology. 2000; 215:554–562.

7. Dean JC, Ilvento CC. Improved cancer detection using computer-aided detection with diagnostic and screening mammography: prospective study of 104 cancers. AJR Am J Roentgenol. 2006; 187:20–28.

8. Berg WA, Gilbreath PL. Multicentric and multifocal cancer: whole-breast US in preoperative evaluation. Radiology. 2000; 214:59–66.

9. Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003; 181:177–182.

10. Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001; 221:641–649.

11. Leconte I, Feger C, Galant C, Berlière M, Berg BV, D'Hoore W, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003; 180:1675–1679.

12. D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology;2013.
13. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73:13–22.

14. Nyström L, Rutqvist LE, Wall S, Lindgren A, Lindqvist M, Rydén S, et al. Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet. 1993; 341:973–978.

15. Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, et al. Efficacy of breast cancer screening by age: new results from the Swedish two-county trial. Cancer. 1995; 75:2507–2517.

16. Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000; 92:1081–1087.

17. Kim SJ, Moon WK, Cho N, Cha JH, Kim SM, Im JG. Computer-aided detection in full-field digital mammography: sensitivity and reproducibility in serial examinations. Radiology. 2008; 246:71–80.

18. van den Biggelaar FJ, Kessels AG, van Engelshoven JM, Boetes C, Flobbe K. Computer-aided detection in full-field digital mammography in a clinical population: performance of radiologist and technologists. Breast Cancer Res Treat. 2010; 120:499–506.

19. Bolivar AV, Gomez SS, Merino P, Alonso-Bartolomé P, Garcia EO, Cacho PM, et al. Computer-aided detection system applied to full-field digital mammograms. Acta Radiol. 2010; 51:1086–1092.

20. Sadaf A, Crystal P, Scaranelo A, Helbich T. Performance of computer-aided detection applied to full-field digital mammography in detection of breast cancers. Eur J Radiol. 2011; 77:457–461.

21. Murakami R, Kumita S, Tani H, Yoshida T, Sugizaki K, Kuwako T, et al. Detection of breast cancer with a computer-aided detection applied to full-field digital mammography. J Digit Imaging. 2013; 26:768–773.

22. Korpraphong P, Limsuwarn P, Tangcharoensathien W, Ansusingha T, Thephamongkhol K, Chuthapisith S. Improving breast cancer detection using ultrasonography in asymptomatic women with non-fatty breast density. Acta Radiol. 2014; 55:903–908.

23. Yang SK, Moon WK, Cho N, Park JS, Cha JH, Kim SM, et al. Screening mammography-detected cancers: sensitivity of a computer-aided detection system applied to full-field digital mammograms. Radiology. 2007; 244:104–111.

24. Kim SH, Kim MH, Oh KK. Analysis and comparison of breast density according to age on mammogram between Korean and Western women. J Korean Radiol Soc. 2000; 42:1009–1014.

25. Kim HY, Seo BK, Kim HY, Yie A, Cho KR, Seol HY, et al. Additional breast ultrasound examinations in clustered calcifications: for improving diagnostic performance. J Breast Cancer. 2009; 12:142–150.

26. Barreau B, de Mascarel I, Feuga C, MacGrogan G, Dilhuydy MH, Picot V, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol. 2005; 54:55–61.

27. Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994; 11:167–180.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download