Abstract
Purpose
There is no standard targeted therapy for the treatment of triple-negative breast cancer (TNBC). Therefore, its management heavily depends on adjuvant chemotherapy. Using core needle biopsy, this study evaluated the histological factors of TNBC predicting the response to chemotherapy.
Methods
One hundred forty-three TNBC patients who received single-regimen neoadjuvant chemotherapy (NAC) with the combination of doxorubicin, cyclophosphamide, and docetaxel were enrolled. The core needle biopsy specimens acquired before NAC were used to analyze the clinicopathologic variables and overall performance of the predictive model for therapeutic response.
Results
Independent predictors of pathologic complete response after NAC were found to be higher number of tumor infiltrating lymphocytes (p=0.007), absence of clear cytoplasm (p=0.008), low necrosis (p=0.018), and high histologic grade (p=0.039). In the receiver operating characteristics curve analysis, the area under curve for the combination of these four variables was 0.777.
Conclusion
The present study demonstrated that a predictive model using the above four variables can predict therapeutic response to single-regimen NAC with the combination of doxorubicin, cyclophosphamide, and docetaxel in TNBC. Therefore, adding these morphologic variables to clinical and genomic signatures might enhance the ability to predict the therapeutic response to NAC in TNBC.
Triple-negative breast cancer (TNBC) is a molecular subtype of breast cancer known for its poor prognosis due to aggressive biologic behavior with a high rate of metastasis and short relapse-free survival unlike luminal or human epidermal growth factor receptor 2 type breast carcinomas; it has no therapeutic targets, making chemotherapy the only option for adjuvant treatment.
After the GEPARDUO trial reported the therapeutic benefit of using a combination treatment regimen that consisted of doxorubicin and cyclophosphamide sequentially administered with docetaxel, this regimen has become a standard therapeutic strategy for neoadjuvant systemic therapy in patients with operable breast cancers [1]. Although the retrospective analyses of clinical trials have reported a greater sensitivity to cytotoxic chemotherapy in TNBC compared to hormone receptor positive breast cancer [23], the pathologic complete response (pCR) rate was still low (28%–47%) [456]. Therefore, the need for a marker that can predict the response to a particular cytotoxic regimen, especially before neoadjuvant chemotherapy (NAC), is becoming even more necessary for the optimization of therapeutic efficacy and prevention of unnecessary complications caused by systemic therapy.
To date, various predictive models and parameters for chemotherapeutic response have been reported, especially based on transcriptional gene signatures; however, implementing them into routine practice has been challenging owing to a lack of consistency and reproducibility caused by tumor heterogeneity, particularly in TNBC. Histomorphological phenotypes reflect the final product of the central dogma from transcription to posttranscriptional modification pathways and they can be used as consistent predictive markers. In our previous studies, particular phenotypes based on histologic characteristics were proven strong predictors of lymph node metastasis in different types of carcinomas including breast [7], colorectum [8], and thyroid [910].
Presently, preoperative core needle biopsy (CNB) is the gold standard procedure in cancer diagnostics. In addition to its diagnostic role, recent data have suggested another role for CNB in the analysis of predictive biomarkers, particularly utilizing histomorphological characteristics [111213]. This study evaluated the capacity of the histological characteristics observed in CNB specimens for predicting the response to combination chemotherapy with doxorubicin, cyclophosphamide, and docetaxel in TNBC patients. To our knowledge, studies reporting the role of the phenotypic features of CNB specimens in predicting response to chemotherapy have been limited in TNBC patients, and this is the first study to suggest a novel use for CNB beyond its standard diagnostic use in breast cancer.
The study cohort included 143 patients with operable TNBC who received preoperative NAC followed by surgical resection at Seoul National University Hospital between January 2009 and December 2014. The study inclusion criteria were as follows: (1) patients who received four to six cycles of NAC using doxorubicin with cyclophosphamide followed by docetaxel combination therapy (doxorubicin, 50 mg/m2; cyclophosphamide, 600 mg/m2; docetaxel, 75 mg/m2); (2) CNB samples with at least four well-preserved cores; and (3) patients with available corresponding surgical specimens to determine the histological response. The clinicopathological parameters were reviewed and collected via an electronic medical records system. Clinical T stage was determined using magnetic resonance imaging data obtained before chemotherapy. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: 1311-102-537).
All hematoxylin and eosin-stained slides for surgical and CNB specimens were retrospectively reviewed by two breast pathologists (Y.Y.J. and H.S.R.) who were blinded to the clinical details, and chemotherapeutic response to therapy was assessed based on CTNoeBC [14]. The pCR was defined as having no evidence of residual invasive carcinoma in both the breast tissue and regional lymph nodes determined by the analyses of the whole sections from primary tumors resected after chemotherapy. Residual carcinoma in situ was not taken into consideration for response assessment in the primary site.
The histological variables that were evaluated to determine the significant predictors of chemotherapeutic response using the CNB specimens were the histological grade of the CNB specimens, the percentage of the area occupied by the tumor infiltrating lymphocytes (TILs), retraction artifact status, small-cell like feature status, level of tumor necrosis, and clear cytoplasm status. The histological grade of the CNB specimen was determined before the administration of the cytotoxic agent according to the Nottingham grading system [15]. The percentage of the area occupied by the TILs over the total intratumoral stromal area was estimated [16]. This percentage was further divided into four categories as follows: none, no infiltration of lymphocytes; mild, <30%; moderate, 30% to 60%; and severe, >60% for lymphocytic infiltration (Figure 1) [17]. Based on this categorization, the four subgroups were further dichotomized into low TIL (none or mild) and high TIL (moderate or severe) groups. Intratumoral stromal TIL was defined as lymphocytes in the stroma between tumor cells without direct contact with the malignant cells according to the international TILs working group [16]. Additionally, we defined "peritumoral TIL" as the lymphocytes surrounding the peripheral border of the tumors as shown in Figure 2A. The relative proportions of peritumoral TIL and stromal TIL were determined. Tumors were considered intratumoral stromal TIL-dominant if the intratumoral stromal TILs outnumbered the peritumoral TILs, and peritumoral TIL-dominant if the peritumoral TILs outnumbered the intratumoral stromal TILs.
The retraction artifact was defined as the existence of clear spaces that separate the tumor cells from the adjacent stroma without endothelial linings. A tumor was defined as having a retraction artifact if it had more than 20% of the tumor cells occupying the retraction artifact (Supplementary Figure 1A, available online) [18].
The small cell-like feature was defined as small hyperchro-matic nuclei and scanty cytoplasm with crush artifact in tumor cells (Supplementary Figure 1B, available online) [19]. A tumor was considered to have a small cell-like feature when more than 10% of the entire tumor area was occupied by cells having the defined phenotype [7].
Tumor necrosis was defined based on the presence of tumor cell nests with eosinophilic debris accompanied by karyorrhexis and pyknosis [20]. A tumor was considered to have necrosis when at least one area with recognizable geographic necrosis was identified. The degree of necrosis was microscopically assessed and divided into four categories, none, focal, partly, and diffuse, which were further dichotomized into the low (none or focal) and high (partly or diffuse) groups (Figure 2B) [13].
Tumors with clear cytoplasm appeared as sheets of polyhedral cells with well-defined cell membranes and clear cytoplasms (Figure 2C). A tumor was defined to have a clear cytoplasm when ≥10% of the tumor cells in the entire core had clear cytoplasms. Ductal carcinoma in situ, fat invasion, and lymphocytes in normal mammary glands were considered present if they were found in more than one area of the given core.
Correlations between the clinicopathologic parameters and response to NAC were evaluated by univariate logistic regression analysis, and a multivariate logistic regression analysis was performed to identify the independent predictive factors. Receiver operating characteristics curves were constructed using the independent predictive factors identified by multivariate analysis, and the area under the curve (AUC) was estimated for all variables, to determine the optimal cutoff values for all possible combinations of the variables. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS version 21.0 (IBM Corp., Armonk, USA).
In the cohort of patients with TNBC, the median age was 46 (31–68) years. The histological grade of the pre-NAC specimens was 2 in 71 patients (49.7%) and 3 in 72 patients (50.3%). Nine cases (6.3%) were clinical stage 1, 82 (57.3%) were clinical stage 2, and 52 (36.4%) were clinical stage 3 (Table 1).
Out of 143 patients, 66 patients (46.2%) achieved pCR to NAC while 77 (53.8%) failed to show pCR. The results of the univariate analysis performed to determine the correlations between the clinicopathologic parameters and response to NAC are summarized in Table 1. The factors showing significant associations with pCR to NAC included low clinical T stage (p=0.038), high histologic grade (p=0.002), high number of TILs (p=0.003), low degree of necrosis (p=0.001), absence of clear cytoplasm (p=0.009), and dominance of intratumoral TILs over peritumoral TILs (p=0.028). Multivariate analysis identified high TIL (OR, 3.484; p=0.007), absence of clear cytoplasm (OR, 0.305; p=0.008), low degree of necrosis (OR, 0.359; p=0.018), and high histological grade (OR, 2.458; p=0.039) as independent predictors of pCR (Table 2).
We designed a predictive model for response to NAC using different combinations of the clinicopathologic variables that had been identified as independent predictors by multivariate logistic regression analysis. These variables included the histological grade, percentage of TILs, degree of necrosis, and small-cell feature. The AUC for the combination of the four variables was 0.777 (Figure 3).
Breast cancer is a group of heterogeneous diseases with numerous genetic alterations yet relatively uniform histological phenotypes. Therefore, identification of the histological characteristics that can help predict the therapeutic response or the clinical prognosis in CNB specimens can prove valuable.
In the present study, we reviewed the histological characteristics routinely encountered in CNB specimens and investigated their capacity to predict the therapeutic response to combination therapy with Adriamycin, cyclophosphamide, and docetaxel, which is the standard cytotoxic regimen most widely administered in TNBC. As a result, four distinctive pathologic variables of TNBC including the histological grade, and the statuses of TIL, necrosis, and clear cytoplasm in tumor cells were identified as strong predictors of the response to NAC.
Recent subgroup analyses of NAC clinical trials have reported a relationship between the histologic grade and therapeutic sensitivity to pCR in TNBC [2122]. In the Spanish Breast Cancer Research Group (GEICAM)/2006-03 Core-Basal phase II clinical trial, even though none of the clinicopathologic variables or gene signatures evaluated were identified as significant predictors of pCR [22], there was a strong correlation between high histological grade and cytotoxic effects of NAC, which is consistent with the results of the present study. In our opinion, the higher pCR rate in tumors with high histologic grades might be attributable to the increased mitotic index, which is consistent with previous results showing that increased mitotic activity had a positive predictive role in breast cancer patients who received NAC [2324]. In addition to high histological grade, tumor necrosis is another histological feature known to be associated with poor breast cancer-specific overall survival, particularly in patients with TNBC [25]. Necrosis, an indicator of tumor hypoxia or low oxygen saturation around the tumor that ultimately results in resistance to chemotherapy [26], has also been suggested as a predictor of response to NAC. In the NAC setting of the present study, tumor necrosis was a negative predictor of the response to NAC, which is consistent with the results of previous studies [1327].
Furthermore, we identified clear cytoplasm as an additional histological feature of tumor cells that can help predict the response to NAC. Although there is no clear evidence for the predictive role of clear cytoplasm in invasive mammary carcinoma, a predominant clear cytoplasm feature was strongly associated with response to chemotherapy in ovarian carcinoma (another prevalent malignancy in females) [28]. Itamochi et al. [28] suggested that carcinomas with clear cell features showed distinct clinicopathologic behaviors that require new therapeutic strategies using specific targeted agents instead of conventional cytotoxic agents.
Lymphocytic infiltration in tumors is another histomorphological characteristic of TNBC that is under investigation to develop a novel therapeutic strategy for the treatment of patients with TNBC. In the present study, tumor infiltration of the lymphocytes was assessed in three steps. First, the percentage of TILs was evaluated based on the recommendations from the international TILs working group [16]. Patients were categorized into four groups as previously suggested by Schalper et al. [17], and subsequently re-categorized into two groups. As a result, a significant association between the percentage of TILs and positive response to combination therapy with adriamycin, cyclophosphamide, and docetaxel was identified. These cytotoxic agents were previously reported to enhance the chemotherapeutic response in breast cancer via various mechanisms including tumor cell sensitization to T-cell mediated cytotoxicity [2930]. Docetaxel reinforces antigen-specific cytotoxic T lymphocyte-mediated tumor cell cytotoxicity, which was also observed in the MCF-7 cell line [29]. In a murine breast cancer model, doxorubicin enhanced the cytotoxic effect of cytotoxic T cells and natural killer cells by eliminating the myeloid-derived suppressor cells [30]. These immunomodulatory effects of the chemotherapeutic agents underlie the correlation between the amount of TILs and chemosensitivity. In addition to the effect of the presence of TILs, we evaluated the role of the location of TILs; pCR rate was significantly higher when the lymphocytic infiltration to the intratumoral stroma outnumbered that to the peritumoral stromal area. To our knowledge, there have been no studies evaluating the role of TILs in the tumor border for TNBC. Further studies are warranted to determine the function of TILs in different locations of infiltration.
In conclusion, we propose a novel predictive model of therapeutic response to the standard NAC regimen in TNBC using CNB specimens, a routine diagnostic tool employed in daily practice. The combined predictive model using four histological variables including the histologic grade, tumor necrosis, TILs, and clear cell feature were identified as relatively good predictors of response to NAC. The results of the present study might be utilized in a predictive model for therapeutic response in patients with TNBC who receive standard NAC with adriamycin, cyclophosphamide, and docetaxel.
Figures and Tables
Figure 1
Representative microphotographs of tumor infiltrating lymphocytes (TIL) according to TIL grade (H&E stain, ×200). (A) None; almost no lymphocytes are present. (B) Mild; only a few lymphocytes infiltrate the tumor stroma. (C) Moderate; moderate lymphocytic infiltration in the stroma. (D) Severe; dense infiltration of lymphocytes surrounding the tumor nests.
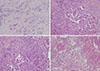
Figure 2
Representative histopathologic parameters. (A) Peritumoral tumor infiltrating lymphocytes (TIL); the lymphocytes surrounding the peripheral tumor border. The border between the tumor nests and the inner stroma is demarcated by the black line, and lymphocytes in the stroma surrounded by the line are considered to be stromal TIL (H&E stain, ×200). (B) The left half of the core biopsy specimen is totally necrotized. Diffuse necrosis (H&E stain, ×100). (C) Tumor cells with clear cytoplasm (H&E stain, ×400).

Figure 3
Receiver operating characteristics analysis for prediction of pathologic complete response to neoadjuvant chemotherapy. AUC=area under the curve.

Table 1
Univariate analysis of pathologic complete response and clinicopathologic parameters

Notes
References
1. von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005; 23:2676–2685.

2. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008; 371:29–40.

3. Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006; 295:1658–1667.

4. Bear HD, Tang G, Rastogi P, Geyer CE Jr, Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012; 366:310–320.

5. Torrisi R, Balduzzi A, Ghisini R, Rocca A, Bottiglieri L, Giovanardi F, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008; 62:667–672.

6. Nabholtz JM, Abrial C, Mouret-Reynier MA, Dauplat MM, Weber B, Gligorov J, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol. 2014; 25:1570–1577.

7. Yoo SH, Park IA, Chung YR, Kim H, Lee K, Noh DY, et al. A histomorphologic predictive model for axillary lymph node metastasis in preoperative breast cancer core needle biopsy according to intrinsic subtypes. Hum Pathol. 2015; 46:246–254.

8. Ryu HS, Kim WH, Ahn S, Kim DW, Kang SB, Park HJ, et al. Combined morphologic and molecular classification for predicting lymph node metastasis in early-stage colorectal adenocarcinoma. Ann Surg Oncol. 2014; 21:1809–1816.

9. Jung YY, Lee CH, Park SY, Park HJ, Min HS, Won JK, et al. Characteristic tumor growth patterns as novel histomorphologic predictors for lymph node metastasis in papillary thyroid carcinoma. Hum Pathol. 2013; 44:2620–2627.

10. Chung YJ, Lee JS, Park SY, Park HJ, Cho BY, Park SJ, et al. Histomorphological factors in the risk prediction of lymph node metastasis in papillary thyroid carcinoma. Histopathology. 2013; 62:578–588.

11. Acs G, Paragh G, Chuang ST, Laronga C, Zhang PJ. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol. 2009; 33:202–210.

12. Wang J, Buchholz TA, Middleton LP, Allred DC, Tucker SL, Kuerer HM, et al. Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer. 2002; 94:3107–3114.

13. Pu RT, Schott AF, Sturtz DE, Griffith KA, Kleer CG. Pathologic features of breast cancer associated with complete response to neoadjuvant chemotherapy: importance of tumor necrosis. Am J Surg Pathol. 2005; 29:354–358.

14. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.

15. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.

16. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–271.

17. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014; 20:2773–2782.

18. Acs G, Dumoff KL, Solin LJ, Pasha T, Xu X, Zhang PJ. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol. 2007; 31:129–140.

20. Kumar V. AK Abbas. In : Aster JC, editor. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia: Elsevier Saunders;2015.
21. Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011; 305:1873–1881.

22. Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting: results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012; 136:487–493.

23. Kim KI, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014; 17:40–46.

24. Zhang G, Xie W, Liu Z, Lin C, Piao Y, Xu L, et al. Prognostic function of Ki-67 for pathological complete response rate of neoadjuvant chemotherapy in triple-negative breast cancer. Tumori. 2014; 100:136–142.

25. Rubovszky G, Horváth Z, Tóth E, Láng I, Kásler M. Significance of histomorphology of early triple-negative breast cancer. Pathol Oncol Res. 2012; 18:823–831.

26. Ueda S, Roblyer D, Cerussi A, Durkin A, Leproux A, Santoro Y, et al. Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res. 2012; 72:4318–4328.

27. Patel T, Gupta A, Shah M. Pathological predictive factors for tumor response in locally advanced breast carcinomas treated with anthracyclin-based neoadjuvant chemotherapy. J Cancer Res Ther. 2013; 9:245–249.

28. Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008; 99:653–658.

SUPPLEMENTARY MATERIAL
Supplementary Figure 1
Representative microphotographs of histopathological parameters; retraction artifact and small cell-like feature. (A) Note the clear spaces that separate the tumor cells from the adjacent stroma without endothelial linings. Retraction artifact (H&E stain, ×200). (B) Note the tumor cells of small hyperchromatic nuclei and scanty cytoplasm with crush artifact. Small cell-like feature (H&E stain, ×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download