Abstract
The prognosis associated with brain metastasis arising from breast cancer is very poor. Eribulin is a microtubule dynamic inhibitor synthesized from halichondrin B, a natural marine product. In a phase III study (EMBRACE), eribulin improved overall survival in patients with heavily pretreated metastatic breast cancers. However, these studies included few patients with brain metastases. Metastatic brain tumors (MBT) were detected during first-line palliative chemotherapy in a 43-year-old woman with breast cancer metastasis to the lung and mediastinal nodes; the genetic subtype was luminal B-like human epidermal growth factor receptor 2 (HER2)-negative. Whole brain radiotherapy (WBRT) followed by eribulin treatment continuously decreased the size, and induced regression, of the MBT with systemic disease stability for 12 months. Another 48-year-old woman with metastatic breast cancer (HER2+ subtype) presented with MBT. Following surgical resection of the tumor, eribulin with concurrent WBRT showed regression of the MBT without systemic progression for 18 months.
Brain metastasis from breast cancer is less common than metastasis to the bone, lung, and liver. However, the prognosis of brain metastasis is very poor, with a median survival time ranging between 5.4 and 13 months. Additionally, it is usually discovered at a later stage during the systemic progression of breast cancer [12]. While brain metastasis is generally observed in 10% to 20% of patients with metastatic breast cancers (MBC), autopsy series have revealed that it is, in fact, present in >34% of patients [13]. Current therapies for brain metastasis include whole brain radiotherapy (WBRT), surgery, stereotactic radiation therapy (SRT), corticosteroids, and systemic chemotherapy. WBRT is considered the standard treatment for metastatic brain tumors (MBT); the role of chemotherapy is still controversial owing to the difficulties associated with passage through the blood-brain barrier (BBB) [12].
Eribulin is a microtubule dynamic inhibitor synthesized from halichondrin B, a natural marine product [4]. Halichondrin B binds to tubulin in the vinca domain, thereby inhibiting tubulin polymerization. However, unlike other tubulin polymerization inhibitors, halichondrin B does not function by inhibiting tubulin growth or by shortening the microtubule [56]. Eribulin is currently acknowledged as a new line of therapy for MBC [7]. In a phase III study (eribulin monotherapy versus treatment of physician's choice in patients with MBC [EMBRACE]), eribulin treatment improved overall survival in patients with heavily pretreated MBC, but these studies included few patients with brain metastases [8]. Therefore, it is crucial to evaluate the effectiveness and safety of eribulin in the treatment of patients with breast cancer with brain metastasis.
Herein, we report two cases of breast cancer with brain metastasis that were treated with eribulin mesylate combined with local treatment for MBT.
MBT were found during first-line palliative chemotherapy (docetaxel plus capecitabine) in a 43-year-old woman with breast cancer with metastasis to the lung and the mediastinal nodes, in Inje University Busan Paik Hospital in June 2013. The patient complained of headache and tinnitus but there were no neurologic symptoms. Magnetic resonance imaging (MRI) of her brain showed two metastatic lesions with edema at the cerebrum; the larger lesion measured 2.7×2.1 cm (Figure 1A).
Five years earlier, in July 2008, the patient had undergone a left modified radical mastectomy for invasive ductal carcinoma. The pathologic stage of the disease was T3N3M0 and the histologic grade was low. The genetic subtype was luminal B-like human epidermal growth factor receptor 2 (HER2)-negative, which was positive for estrogen receptor (ER) and progesterone receptor (PR), negative for HER2, and the Ki-67 labeling index >14%. The patient received adjuvant chemotherapy with doxorubicin and cyclophosphamide followed by paclitaxel (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2, every 3 weeks, intravenously for four cycles; followed by paclitaxel 175 mg/m2, every 3 weeks, intravenously for four cycles). Following the chemotherapy treatment, she received radiation therapy and tamoxifen.
Four years after the surgery, in May 2012, positron emission tomography (PET) revealed metastases in the patient's right lung, and in her paratracheal, subcarinal, and right supraclavicular lymph nodes, although she showed no symptoms or signs of either local or distant metastatic recurrence. A needle biopsy confirmed the presence of metastatic lesions from breast cancer in the right supraclavicular lymph node. The genetic subtype of the metastatic node was luminal B-like HER2-negative (ER-positive, PR-negative, HER2-negative, and Ki-67 labeling index >14%). Based on the first-line palliative chemotherapy, the patient received docetaxel (75 mg/m2, every 3 weeks, intravenously) plus capecitabine (1,250 mg/m2 bis in die, per os [p.o.], on days 1–14, with a 1-week withdrawal period, every 3 weeks) for 15 cycles.
The MBT were detected during the docetaxel plus capecitabine chemotherapy in June 2013 (Figure 1A). The patient underwent γ-knife SRT and received CMF (cyclophosphamide 100 mg/m2 p.o., on days 1–14, methotrexate 40 mg/m2 intravenously on days 1 and 8, and 5-fluorouracil 600 mg/m2 intravenously on days 1 and 8, every 4 weeks) for four cycles as second-line palliative chemotherapy. The 4-month follow-up brain MRI showed that the metastatic lesion had decreased in size from 2.7 to 2.5 cm (Figure 1B), but the PET-computed tomography (CT) image showed increased glucose uptake and size in the metastatic lymph nodes of the mediastinum, indicating aggravation.
The patient received WBRT (total 3,000 cGy: 300 cGy/day for 10 days). She then received eribulin single therapy (1.4 g/m2 on days 1 and 8, intravenously, every 3 weeks) 4 days later as third-line palliative chemotherapy in November 2013. At the 3-month follow-up appointment, after the fourth cycle of eribulin, the MBT had further decreased in size; the heterogeneous enhancing lesion decreased from 2.5 to 1.7 cm. Additionally, the oval-shaped cystic lesion had decreased from 1.5 to 0.8 cm (Figure 2A). After the sixth cycle of eribulin treatment, the patient discontinued use owing to financial reasons; the eribulin therapy was restarted 3 months later. At the 8-month follow-up examination in July 2014, after the eighth cycle of eribulin treatment, the MBT showed regression with systematically stable disease (Figure 2B). The patient was still receiving eribulin and there were no severe hematologic or non-hematologic adverse events reported during or after the chemotherapy. At the 10-month follow-up examination in September 2014, following the tenth cycle of eribulin treatment, a PET-CT scan showed that the mediastinal nodes had increased in size and that glucose metabolism in these nodes had also increased. The MBT progressed systemically 12 months after eribulin single therapy in November 2014.
As the patient had the luminal B breast cancer subtype, and she was postmenopausal, we initiated treatment with an aromatase inhibitor (letrozole) in December 2014. In July 2015, we changed the regimen to everolimus plus exemestane due to systemic progression. The patient is still alive, without any neurologic symptoms, more than 24 months following the MBT diagnosis.
In May 2012, a 47-year-old woman visited the Breast Cancer Center of Gangnam Severance Hospital for evaluation of an ulcerofungating mass involving her right whole breast. She was diagnosed with stage IV invasive ductal carcinoma (cT4N3M1), which had metastasized to distant organs including the liver and bone. Pathological examination via immunohistochemistry showed that the tumor was ER- and PR-negative, and HER2-positive. Primary systemic chemotherapy, consisting of six cycles of docetaxel (75 mg/m2, every 3 weeks, intravenously) and doxorubicin (50 mg/m2, every 3 weeks, intravenously) was administered from May 2012 to August 2012. Salvage mastectomy for wound management was performed in September 2012. Two months after completion of the first-line treatment, a follow-up liver CT showed progression of the hepatic metastasis with increased tumor number and size. As a second-line systemic treatment, paclitaxel (75 mg/m2, every 3 weeks, intravenously) and trastuzumab (8 mg/kg loading dose and 6 mg/kg, every 3 weeks, intravenously) were administered. In May 2013, a liver CT revealed that the metastatic lesions in the liver had progressed despite the six cycles of paclitaxel and trastuzumab. The patient was treated with lapatinib (1,250 mg, daily, p.o.) and capecitabine (2,000 mg/m2, on days 1–14, every 3 weeks, p.o.) as third-line chemotherapy. A partial response was achieved.
Despite this partial response, brain metastasis developed after 20 cycles of lapatinib and capecitabine. On July 20, 2014, the patient visited the emergency department with right-sided weakness. A brain MRI identified a 2.5-cm metastatic lesion on her left frontal-parietal lobe (Figure 3A). Craniotomy and surgical resection of the tumor was successfully performed on July 21, 2014. At the time of the brain metastasis, a newly developed lesion was found in the S8 area of the liver on CT performed on July 29, 2014. After tumor removal, systemic therapy with eribulin (1.4 g/m2, on days 1 and 8, every 3 weeks, intravenously) was started in August 2014. For further local treatment of the brain metastasis, the patient received WBRT (total 3,000 cGy: 250 cGy/day for 12 days) with a boost dose to the tumor bed (total 3,600 cGy: 300 cGy/day for 12 days). During the treatment period with eribulin, local wound dehiscence developed at the site of craniotomy in the scalp. To manage this, a local skin flap surgery was performed, and the eribulin treatment was temporally halted for 4 weeks. As of December 2015, the patient was on her 23rd cycle of eribulin, without tumor recurrence or progression in the brain or liver. Her last follow-up studies, including brain MRI and liver CT, carried out on November 20, 2015 revealed stable disease (Figure 3B).
The clinical regression, without symptoms, of MBT from breast cancer was sustained for 24 and 18 months in case 1 and case 2, respectively, using multimodality therapies that included surgery, SRT, WBRT, chemotherapy, and endocrine therapy. WBRT followed by eribulin induced a continuous decrease in the size of the MBT with systemic disease stability for 12 months in case 1. Surgery followed by eribulin with concurrent WBRT inhibited systemic progression for 18 months in case 2.
In order to effectively treat MBT, a chemotherapeutic agent should induce effective cytotoxicity in the tumor cells. It should be able to reach adequate concentrations, maintain this concentration in the tumor's extracellular space, and cross the BBB while evading active efflux mechanisms [2]. Compared with that of primary brain tumors, the BBB of MBT tends to be relatively permeable [9]. In addition, the expression of P-glycoprotein (P-gp), which is associated with permeability of the BBB, is decreased in MBT [10]. Furthermore, the permeability of the BBB can be increased by WBRT [1112].
In line with our experience, a previous case report showed that eribulin therapy may be beneficial in the treatment of MBT arising from breast cancer [13]. In a patient with progression of brain and liver metastasis after WBRT followed by lapatinib and capecitabine treatment, eribulin achieved a significant response in the MBT 1 month later and this effect lasted for 4 months.
The following hypotheses could explain the mechanism of the therapeutic effect of eribulin on MBT in our cases: first, eribulin was efficacious against the systemic disease; second, WBRT may have facilitated the passage of eribulin across the BBB by decreasing the function of P-gp; third, the antiangiogenic action of eribulin could have increased the transmission of eribulin itself. A new mode of action for eribulin that involves remodeling of the tumor vasculature through a novel antivascular activity has already been reported [14].
Eribulin may offer a clinical benefit in the treatment of MBT arising from breast cancer. WBRT followed by eribulin, or eribulin with concurrent WBRT may represent an effective treatment option for breast cancer patients with brain metastasis. Future studies are warranted to evaluate the precise effect of eribulin and its mechanism of action on brain metastasis.
Figures and Tables
Figure 1
Magnetic resonance imaging in case 1. (A) A heterogeneous enhancing brain parenchymal mass with hemorrhagic component (2.7×2.1 cm) and a rim enhancing cystic mass (1.5×1.2 cm) were identified on right frontoparietal region of the cerebrum in June 2013. (B) Response of metastatic brain tumors to gamma knife stereotactic radiation therapy in October 2013. The metastatic lesion had decreased in size from 2.7 to 2.5 cm. A cystic lesion in anterior portion showed no gross interval change (1.5×1.2 cm)
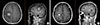
Figure 2
Magnetic resonance imaging after whole brain radiotherapy followed by eribulin in case 1. (A) The posterior heterogeneous enhancing lesion and anterior oval shaped cystic lesion had continuously decreased respectively (2.5 to 1.7 cm and 1.5 to 0.8 cm) in January 2014. (B) The heterogeneous enhancing lesion and oval shaped cystic mass had regressed respectively (1.7 to 1.2 cm and 0.8 to 0.6 cm) in July 2014.
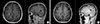
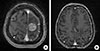
Figure 3
Magnetic resonance imaging after surgery followed by eribulin with concurrent whole brain radiotherapy in case 2. (A) A 2.5 cm metastatic lesion with edema was identified on her left frontal-parietal lobe in July 2014. (B) No tumor recurrence showed in her brain after treatment in November 2015.
References
2. Arslan C, Dizdar O, Altundag K. Chemotherapy and biological treatment options in breast cancer patients with brain metastasis: an update. Expert Opin Pharmacother. 2014; 15:1643–1658.

3. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma: autopsy study. Cancer. 1983; 52:2349–2354.

4. Shablak A. Eribulin for advanced breast cancer: a drug evaluation. J Breast Cancer. 2013; 16:12–15.

5. Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin: discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991; 266:15882–15889.

6. Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005; 4:1086–1095.

7. Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roché H, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010; 28:3922–3928.

8. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011; 377:914–923.

9. Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992; 141:1115–1124.
10. Tóth K, Vaughan MM, Peress NS, Slocum HK, Rustum YM. MDR1 P-glycoprotein is expressed by endothelial cells of newly formed capillaries in human gliomas but is not expressed in the neovasculature of other primary tumors. Am J Pathol. 1996; 149:853–858.
11. Bart J, Nagengast WB, Coppes RP, Wegman TD, van der Graaf WT, Groen HJ, et al. Irradiation of rat brain reduces P-glycoprotein expression and function. Br J Cancer. 2007; 97:322–326.

12. Mima T, Toyonaga S, Mori K, Taniguchi T, Ogawa Y. Early decrease of P-glycoprotein in the endothelium of the rat brain capillaries after moderate dose of irradiation. Neurol Res. 1999; 21:209–215.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download