Abstract
Purpose
To evaluate imaging features of pure lobular carcinoma in situ (LCIS) on magnetic resonance imaging (MRI) in patients who underwent immediate re-excision after lumpectomy.
Methods
Twenty-six patients (46.1±6.7 years) with 28 pure LCIS lesions, who underwent preoperative MRI and received curative surgery at our institution between 2005 and 2013, were included in this study. Clinicopathologic features associated with immediate re-excision were reviewed and analyzed using Fisher exact test or the Wilcoxon signed rank test.
Results
Of the 28 lesions, 21.4% (6/28, six patients) were subjected to immediate re-excision due to resection margin involvement by LCIS. Nonmass lesions and moderate-to-marked background parenchymal enhancement on MRI were more frequently found in the re-excision group than in the single operation group (100% [6/6] vs. 40.9% [9/22], p=0.018; 83.3% [5/6] vs. 31.8% [7/22], p=0.057, respectively). The median lesion size discrepancy observed between magnetic resonance images and histopathology was greater in the re-excision group than in the single operation group (-0.82 vs. 0.13, p=0.018). There were no differences in the mammographic or histopathologic findings between the two groups.
Lobular carcinoma in situ (LCIS), a noninvasive proliferation of lobular cells within a terminal-duct lobular unit, involves multicentric lobules and both the breasts in 85% and 30% to 67% of patients, respectively [12]. It is also found in 0.5% to 4% of otherwise benign breast biopsies, and a precursor-product relationship exists between LCIS and invasive lobular carcinoma [23]. Although early studies reported that both breasts are at equal risk for later cancer development, more recent study has reported that approximately two thirds of subsequent carcinomas develop in the ipsilateral breast [4]. The relative risk for subsequent development of invasive carcinoma in patients with LCIS ranges from 4 to 12 times that in women without LCIS. Previous studies have shown that when lobular neoplasia is found on image-guided core biopsy, the upgrade rate varies from 2% to 40%. Thus, the National Comprehensive Cancer Network guidelines currently recommend excisional biopsy in LCIS cases [5].
In our institution, owing to the increased use of supplemental ultrasonography (US) screening and magnetic resonance imaging (MRI), the incidence of LCIS lesions after image-guided core needle biopsy and subsequent surgical excision has increased. Furthermore, over the last decade, there have been a substantial number of re-excision cases due to resection margin involvement by LCIS. Thus, the objective of this study was to evaluate the imaging features of pure LCIS on MRI in patients who underwent immediate re-excision after lumpectomy.
This study was approved by our Institutional Review Board (IRB 1410-099-619), and the requirement for informed consent was waived. Between 2005 and 2013, a total of 21,270 consecutive patients underwent image-guided percutaneous breast biopsy at our hospital. We searched the pathology database for patients with a pathological diagnosis of LCIS; 60 such patients were found. Among them, 45 patients underwent surgical excision. At the discretion of the patient or the clinician, 15 patients did not undergo surgical excision. Of the 45 patients who underwent excision, 11 had concurrent malignant disease (five cases of ductal carcinoma in situ, three cases of invasive lobular carcinoma, and three cases of invasive ductal carcinoma); preoperative magnetic resonance (MR) images were not available for five patients; and the 2-year follow-up information was not available for one patient. Finally, a total of 28 LCIS lesions in 26 patients (46.1±6.7 years) who had undergone surgical excision after mammography and MRI were included. One case of bilateral LCIS and one case of recurrent LCIS in the ipsilateral breast were included in the study.
Mammographic images were obtained using a Senographe 2000D instrument (GE Healthcare, Milwaukee, USA) or a Lorad Selenia instrument (Hologic, Bedford, USA). MR examinations were performed using a 1.5-T system (Signa; General Electric Medical Systems, Milwaukee, USA) with a dedicated breast coil (8-channel HD Breast Array; General Electric Medical Systems). After obtaining transverse localizer images on both the sides, sagittal fat-suppressed T2-weighted fast spin-echo images were obtained (repetition time [TR]/echo time [TE], variable from 5,500 to 7,150/82; matrix 320×192; field of view, 200 mm×200 mm; 1.5-mm slice thickness; no gap). Dynamic contrast-enhanced examinations included one precontrast and five postcontrast bilateral sagittal image acquisitions using a fat suppressed T1-weighted 3D fast spoiled gradient echo sequence (TR/TE, 6.5/2.5; matrix 320×256; flip angle, 10°; field of view, 200 mm×200 mm; 1.5-mm slice thickness; no gap). Five postcontrast image series were obtained at 91, 180, 360, 449, and 598 seconds after the start of contrast administration. For all studies, early subtraction (i.e., first postcontrast images minus precontrast images), axial reformatted images, and 3D maximum intensity projection images were generated. All available images were reviewed and classified by two radiologists with consensus using the American College of Radiology Breast Imaging Reporting and Data System Atlas lexicon [6]. Lesion size was defined as the maximal diameter of the enhancing lesion on early postcontrast images, and lesion type was categorized as a mass or nonmass enhancement. The level of background parenchymal enhancement was classified as minimal, mild, moderate, or marked, based on the first postcontrast T1-weighted and subtraction images. Initial-phase kinetic features were classified as slow, medium, or fast, by comparing the signal intensity of the first postcontrast images to that of precontrast images. A slow pattern indicated an intensity increase of less than 50%, a medium pattern indicated an intensity increase between 50% and 100%, and a fast pattern indicated an intensity increase of over 100%. The delayed phase kinetic feature was categorized as persistent, plateau, or washout. A persistent pattern indicated an intensity increase of more than 10% when the signal intensity of the delayed postcontrast images was compared with that of the first postcontrast images. A plateau pattern indicated a signal intensity change of less than 10% after peak enhancement. Finally, a washout pattern indicated an intensity decrease of more than 10% after peak enhancement. Lesion size discrepancy was calculated as (lesion size on MRI–lesion size on surgical histopathology)/lesion size on histopathology. Thus, a negative value indicated an underestimation and a positive value indicated an overestimation of the lesion size on MRI.
Eight lesions were detected by screening mammography due to calcifications, 14 lesions were detected by supplemental US screening, and six lesions were detected in contralateral breasts in patients with proven malignancy on preoperative MRI. As surgical excision is routinely recommended for women with a core biopsy diagnosis of LCIS in our institution, all lesions were confirmed through surgical excision.
An experienced pathologist (I.A.P.), with 29 years of experience in breast histopathology, and two radiologists (N.C. and A.J.C.) reviewed all histopathology slides and the mammography and MR imaging findings. On the histopathology slides, they analyzed the histologic type and lesion size of pure LCIS. To exclude coexisting benign pathologies detected by imaging, they correlated the imaging features with pure LCIS features. Lesion size, a location in fibroglandular tissue or the fat layer, and the morphology of the LCIS component on imaging findings were compared with those on the histopathology slides. Based on the histopathologic and surgical reports, the lesions were divided into either the re-excision group or the single operation group. In our institution, when LCIS involvement is detected at the resection margins, subsequent reexcisions are routinely performed. Frozen biopsy samples were not collected during the surgeries.
Histologic type and imaging features were compared between the re-excision group and the single operation group using Fisher exact test or the Wilcoxon signed rank test. SPSS version 19.0 (IBM Corp., Armonk, USA) was used for all statistical analyses and two-tailed p-values of <0.05 were considered to indicate a statistical significance.
The 28 lesions comprised 20 (71.4%) classic type LCIS, six (21.4%) nonclassic type LCIS, and two (7.1%) pleomorphic type LCIS. Immediate re-excision due to resection margin involvement of LCIS was performed for 21.4% of the lesions (6/28) in six patients (Table 1). One recurrent LCIS lesion occurred in a patient's ipsilateral breast 28 months after the initial operation (Table 1, cases 15 and 16). No recurrence was found in the remaining lesions during the median follow-up period of 37 months (range, 7–100 months). On MRI, 100% (28/28) of the lesions showed correlative, suspicious findings, including nonmass enhancements (53.6%, 15/28) or masses (46.4%, 13/28).
On mammography, 53.6% of lesions (15/28) showed negative findings and 46.4% of lesions (13/28) showed correlative, findings suspicious for calcification (n=10) or focal asymmetry (n=3). On MRI, all lesions showed a correlated enhancing lesion; 53.6% (15/28) appeared as nonmass enhancements and 46.4% (13/28) appeared as masses (Table 2). The most common features of nonmass enhancements were a regional distribution (21.4%, 6/28) or a heterogeneous internal enhancement pattern (39.3%, 11/28) (Figure 1). The most common mass features included irregular shape (39.3%, 11/28), irregular margin (25.0%, 7/28), or homogeneous internal enhancement (28.6%, 8/28) (Figure 2). The most common kinetic features were initial fast enhancements (75.0%, 21/28) or delayed persistent kinetics (42.9%, 12/28).
Nonmass lesions on MRI or moderate-to-marked background parenchymal enhancement were more frequently found in the re-excision group than in the single operation group (100% [6/6] vs. 40.9% [9/22], p=0.018; 83.3% [5/6] vs. 31.8% [7/22], p=0.057, respectively) (Table 3, Figures 1, 2). The median discrepancy in lesion size between the MR images and histopathology was greater in the re-excision group than in the single operation group (-0.82 vs. 0.13, p=0.018). No differences between the two groups were observed in the mammographic findings or the histopathologic analysis.
We found that pure LCIS lesions in patients who underwent immediate re-excision after lumpectomy due to resection margin involvement by LCIS tended to show more nonmass lesions or more moderate-to-marked background parenchymal enhancement on breast MRI (100% [6/6] vs. 40.9% [9/22], p=0.018; 83.3% [5/6] vs. 31.8% [7/22], p=0.057, respectively) than the lesions in patients who underwent a single operation.
The American Society of Surgical Oncology has reported that classic LCIS at the resection margin is not an indication for re-excision, but that significant pleomorphic LCIS at the resection margin is uncertain [7]. This guideline was based on a few studies in which the presence of LCIS at the resection margin did not affect subsequent local recurrence [89]. However, another study reported that LCIS or atypical lobular hyperplasia at the margin was significantly associated with increased local recurrence [10]. The latter study reported a recurrence rate of 39% in the positive margin group compared with a recurrence rate of just 7.9% in the negative control group. Furthermore, LCIS shares similar gene expression profiling features with invasive lobular carcinoma [11], supporting a role for LCIS as not only a risk factor for breast cancer, but also as a direct precursor of invasive lobular carcinoma. Thus, we hypothesized that an increased understanding of the imaging features associated with pure LCIS, with a focus on the re-excision group, may help to reduce subsequent local recurrence. Increased awareness would also reduce the number of lumpectomies performed on pure LCIS lesions.
In our institution, LCIS at the margin of a lumpectomy specimen is routinely observed in histopathology reports. In such cases, re-excision is subsequently performed; thus, we were able to evaluate MR imaging features of pure LCIS, as well as features associated with margin involvement by LCIS. Although all included lesions in our study were visible on MRI, 21.4% (6/28) of women underwent immediate re-excision after the initial surgical excision owing to LCIS involvement of the resection margin. The main reason for this re-excision was thought to be an underestimation of the extent of the lesion based on the preoperative MRI. MRI findings of LCIS are generally more subtle than those of ductal carcinoma in situ or other invasive cancers. The majority of nonmass LCIS lesions in our study showed a regional, focal distribution, or heterogeneous internal pattern, while irregular shapes with homogeneous enhancement and delayed persistent kinetics, were observed for masses. These findings are not particularly suspicious and they are contrary to those of the majority of invasive cancers, which show ductal distribution or internal clumped enhancement pattern for nonmass lesions, and irregular shapes with spiculated margins, or marked internal enhancement, for mass lesions [12]. Furthermore, the moderate-to-marked background parenchymal enhancement observed in the re-excision group in our study may limit detection of a lesion or assessment of the extent of the lesion. It has been suggested that physiologic fibroglandular tissue enhancement after intravenous injection of contrast agent associated with hormonal influences may affect the performance of breast MRI [1314]. Indeed, a recent study reported that the most common feature of breast cancers that are not diagnosed during screening is mimicry of physiologic background parenchymal enhancement [15]. Thus, the low conspicuity of nonmass LCIS lesions with moderate-to-marked background parenchymal enhancement could lead to an underestimation of the extent of the lesion and resection margin involvement. In future interpretations of MRI in women with LCIS, lesion size should be carefully measured to obviate underestimation and re-excision. In addition, bracketing of the lesion may be used to determine the extent of the surgery required.
Our study has several limitations. First, our sample size may have been too small to draw a solid conclusion. Second, as we only included cases confirmed by surgical excision to avoid verification bias, there may have been selection bias towards cases with more suspicious imaging features. However, this may not have affected our results.
In conclusion, the majority of pure nonmass LCIS lesions showed regionally, or focally distributed, heterogeneous internal enhancement, while masses were depicted as irregular shapes with homogeneous enhancement and delayed persistent kinetics. Although all of the LCIS lesions were visible on MRI in our study, the associated imaging findings tended to be more subtle than those of invasive cancers. This resulted in an underestimation of lesion extent and a re-excision rate of 21.4% (6/28). Increased awareness of these features will contribute to more accurate assessments of lesion extent on MRI.
Figures and Tables
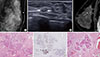 | Figure 1Nonclassic lobular carcinoma in situ (LCIS) in 52-year-old woman who underwent re-excision due to resection margin involvement (case 10 in Table 1). (A) Mediolateral oblique magnification mammogram shows cluster of calcifications. (B) Ultrasonography shows an irregular, indistinct mass with calcifications (arrows). (C) Early contrast-enhanced sagittal magnetic resonance images shows regional, nonmass enhancement (arrows) in the moderate background parenchymal enhancement. (D) Photomicrograph of surgical specimen showing LCIS (arrows) adjacent to radial scar (H&E stain, ×10). (E) Photomicrograph of surgical specimen showing negative staining of E-cadherin in LCIS involving area (immunohistochemistry, ×10). (F) Photomicrograph of pleomorphic LCIS area showing pleomorphic cells and comedonecrosis (arrows) (H&E stain, ×40). The lesion was initially measured as 0.9 cm on magnetic resonance imaging. However, total LCIS extent including re-excision specimen was 8.7 cm. |
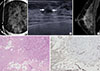 | Figure 2Nonclassic lobular carcinoma in situ (LCIS) in 40-year-old woman who underwent single operation (case 2 in Table 1). (A) Mediolateral oblique magnification mammogram shows negative finding. Skin marker is placed for the mass found on ultrasonography (US). (B) US shows an irregular, indistinct mass (arrows) in the right upper breast. (C) Early contrast-enhanced sagittal magnetic resonance images shows an irregular mass (arrows) with homogeneous enhancement in the marked background parenchymal enhancement. The lesion shows fast rise and delayed washout enhancement pattern. (D) Photomicrograph of surgical specimen shows LCIS involving sclerosing adenosis (arrows) (H&E stain, ×10). (E) Photomicrograph of surgical specimen showing negative staining of E-cadherin in LCIS involving area (immunohistochemistry, ×10). The lesion was initially measured as 1.5 cm on magnetic resonance imaging. Lesion size on surgical histopathology was 2.0 cm. |
Table 1
Clinical and histopathologic features of 28 lobular carcinoma in situ lesions

Table 2
Features of lobular carcinoma in situ on magnetic resonance imaging according to the breast imaging-reporting and data system lexicon

Table 3
Comparison of features of the re-excision and the single operation groups of lobular carcinoma in situ

References
1. Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941; 17:491–496.3.

2. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. Lyon: International Agency for Research on Cancer;2012.
3. Hwang ES, Nyante SJ, Yi Chen Y, Moore D, DeVries S, Korkola JE, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004; 100:2562–2572.

4. Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997; 6:297–301.
5. Clinical practice guidelines in oncology (NCCN Guidelines®). National Comprehensive Cancer Network. Accessed April 25th, 2015. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
6. Morris EA, Comstock CE, Lee CH. ACR BI-RADS® magnetic resonance imaging. In : D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS® Atlas, Breast Imaging and Reporting and Data System. 5th ed. Reston: American College of Radiology;2013.
7. Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014; 32:1502–1506.

8. Sadek BT, Shenouda MN, Abi Raad RF, Niemierko A, Keruakous AR, Goldberg SI, et al. Risk of local failure in breast cancer patients with lobular carcinoma in situ at the final surgical margins: is re-excision necessary? Int J Radiat Oncol Biol Phys. 2013; 87:726–730.

9. Ciocca RM, Li T, Freedman GM, Morrow M. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol. 2008; 15:2263–2271.

10. Apple SK, Matin M, Olsen EP, Moatamed NA. Significance of lobular intraepithelial neoplasia at margins of breast conservation specimens: a report of 38 cases and literature review. Diagn Pathol. 2010; 5:54.

11. Cao D, Polyak K, Halushka MK, Nassar H, Kouprina N, Iacobuzio-Donahue C, et al. Serial analysis of gene expression of lobular carcinoma in situ identifies down regulation of claudin 4 and overexpression of matrix metalloproteinase 9. Breast Cancer Res. 2008; 10:R91.

12. Gutierrez RL, DeMartini WB, Eby PR, Kurland BF, Peacock S, Lehman CD. BI-RADS lesion characteristics predict likelihood of malignancy in breast MRI for masses but not for nonmasslike enhancement. AJR Am J Roentgenol. 2009; 193:994–1000.

13. DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol. 2012; 198:W373–W380.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download