Abstract
Purpose
The aim of this retrospective study was to investigate whether there are prognostically different subgroups among patients with pathologic N3 (pN3) breast cancer.
Methods
The records of 220 patients who underwent surgery for pN3 breast cancer from January 2006 to September 2012 were reviewed. All patients received adjuvant therapy according to standard protocols. The primary outcome was disease-free survival (DFS).
Results
Patients were followed for a median time of 68.3 months after their primary surgery (range, 10–122 months), during which time 75 patients (34.1%) had developed disease recurrence and 48 patients (21.8%) had died. The DFS and overall survival were 67.8% and 86.1%, respectively, at 5 years. Multiple logistic regression analysis showed that young age (<35 years, p=0.009), high serum neutrophil/lymphocyte ratio (>3.0) (p=0.020), high nodal ratio (number of metastatic lymph nodes divided by number of removed nodes) (>0.65) (p=0.062), and molecular phenotype (p=0.012) were significantly associated with tumor recurrence. Tumor biological subtype was the most significant predictor of recurrence. The 5-year DFS rates in patients with hormone receptor (HR) positive and human epidermal growth factor receptor 2 (HER2) negative, HR+HER2+, HR–HER2+, and triple negative subtypes were 82%, 63%, 58%, and 37%, respectively.
Conclusion
Clinical outcomes of patients with extensive nodal metastasis were heterogeneous in terms of prognosis. Tumor biological subtype was the most important prognostic factor for pN3 disease. The prognosis of patients with HR+HER2– subtype in pN3 breast cancer was similar to that of patients with stage II breast cancer.
According to the American Joint Committee on Cancer (AJCC) TNM staging system, breast cancer presenting with positive nodes at the apex of the axilla (level III) and/or 10 or more metastatic lymph nodes (LN) is categorized as pN3 (pathological lymph node status 3) [1]. Approximately 8% to 30% of newly diagnosed breast cancers are at an advanced stage, with pN3 disease showing the worst prognosis among patients with primary breast cancer without distant metastasis [23]. Because patients with advanced-stage breast cancer are thought to be incurable, they either are treated more aggressively or receive suboptimal treatment due to their short life expectancy. Several reports have shown an approximately 40% 5-year overall survival (OS), although this estimate was based on old treatments [45]. However, the 5-year disease-free survival (DFS) and OS of pN3 who underwent surgery with adjuvant therapy (effective chemotherapeutic agents, molecularly targeted agents, and optimal use of endocrine therapy) were reported to have increased to 66% and 81%, respectively [6]. Although there is a possibility of prognosis change with current management, clinical outcomes using the current standard systemic therapy in pN3 breast cancer are currently unknown.
We hypothesized that there are prognostically different subgroups among patients with pN3 breast cancer. This retrospective study was performed to investigate the prognostic factors of pN3 disease.
The study cohort consisted of patients with pN3 breast cancer who underwent surgery at Gachon University Gil Medical Center from January 2006 to September 2012. N3 was defined according to the AJCC seventh TNM staging system as ≥10 axillary nodes on pathology reports and/or ipsilateral supra/infraclavicular LN (SCN/ICN) metastasis and/or ipsilateral internal mammary LN (IMLN) metastasis, as shown by imaging modalities or needle biopsy. A few patients received neoadjuvant chemotherapy (n=7); we excluded this group from the study population. Those with synchronous bilateral breast cancer or metastasis at diagnosis were also excluded. Patients with distant metastasis within 6 months after surgery were excluded. A retrospective search of patient records identified 220 eligible patients. Pathologic data, including tumor size, tumor grade, axillary lymph nodal status, and immunohistochemistry results, were reviewed. Systemic inflammation was assessed by determining serum neutrophil/lymphocyte ratio (NLR), with high and low NLR defined using a cutoff of 3.0 [7]. Lymph node ratio (LNR) was defined as the number of involved nodes divided by the number of LN examined, with high and low LNR defined using a cutoff of 0.65 [8].
The protocol of this retrospective study was reviewed and approved by the Institutional Review Board (approval number: GAIRB 2015-50) of the Gachon University Gil Medical Center. Due to its retrospective design, with little or no risk to study subjects, the Institutional Review Board waived the requirement for informed consent. This study followed the recommendations of the Declaration of Helsinki for biomedical research involving human subjects.
Levels of expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 were evaluated immunohistochemically, as described previously [9]. Mouse monoclonal primary antibodies to ER (1:50), PR (1:50), and Ki-67 (1:50) were obtained from Dako Corporation (Carpinteria, USA). High and low expression of Ki-67 were defined as <20% and ≥20%, respectively [10]. Tumors were regarded as negative for ER and PR when <1% of the tumor cells showed nuclear staining. HER2 was considered negative when the HER2 score was 0 or 1+ by immunohistochemistry, or when the HER2 score was 2+ and without HER2/CEP17 gene amplification as assessed by fluorescence in situ hybridization. The patients were divided into four groups: HR positive/HER2 negative (ER+ and/or PR+, HER2–), HR positive/HER2 positive (ER+ and/or PR+, HER2+), HR negative/HER2 positive (ER– and PR–, HER2+), and triple negative (TN; ER–, PR–, HER2–).
Patients underwent mastectomy or breast-conserving surgery at the discretion of the surgeon. Axillary LN dissection was usually confined to the level I and III axillary LN. All patients received postoperative chemotherapy and radiotherapy. According to the standard protocols of our institution, anthracycline followed by a taxane-based regimen were administered after curative surgery. Adjuvant radiotherapy was administered to 184 patients (83.6%). Tangential fields were used with 50 Gy, at 2 Gy per fraction, irradiation to the breast and chest wall. Approximately 10 to 15 Gy per five fractions boost was added in patients with unresected gross metastasis in the ipsilateral IMLN, and/or ipsilateral SCN/ICN LNs. If hormonal therapy was indicated, it was administered after chemotherapy.
The associations between tumor subtypes and clinicopathologic characteristics were calculated using the chi-square test and Fisher exact test, as appropriate. Multiple stepwise regression analysis was used to assess the association between baseline factors and the risk of recurrence. Disease-free survival (DFS) was defined as the interval from the date of primary surgery to the date of detection of the first locoregional recurrence and/or distant metastasis, or the date of last follow-up. The Kaplan-Meier method and the log-rank test were used for survival analysis. A Cox regression model was utilized to identify independent factors significantly related to disease recurrence. The variables included in the final model were defined by backward selection. Statistical analyses excluded missing and undetermined data. Significance was defined as p<0.05. SPSS version 19.0 software (IBM Corp., Armonk, USA) was used for all statistical analyses.
The clinical and histopathological characteristics of the 220 patients are summarized in Table 1. Median age at diagnosis was 47 years (range, 21–79 years). Thirteen patients (6.0%) were younger than 35 years and 117 (53.2%) were premenopausal. Preoperative SCN/ICN or IMLN metastasis was confirmed by imaging or biopsy in 43 patients (19.5%). The median number of metastatic LN was 15 (range, 5–71). The average number of removed LN was 28.69 (±9.67). Among the 220 patients, 75 (34.1%) showed 20 or more positive LN metastases. Most tumors were stage T1–2 (79.5%), and the mean tumor size was 3.98 cm. After surgery, anthracycline followed by a taxane-based regimen was used in all patients. Of the 220 patients, 143 (65.0%) had hormone receptor-positive tumors, with 140 of these receiving adjuvant hormonal therapy.
Patients were followed for a median of 68.3 months after primary surgery (range, 10–118 months). In all, 14 patients had locoregional recurrence only, whereas 61 developed distant metastasis as the first event, and both were found in 19 patients (8.6%) (Table 2). Recurrence developed within 2 to 3 years after surgery in 44% of those who experienced a recurrence.
During the follow-up period, 75 patients (34.1%) developed disease recurrence, and 48 patients (21.8%) died. The DFS and OS were 72.2% and 86.1% at 5 years, respectively (Figure 1).
The prognostic factor analysis for DFS is shown in Table 3. Age younger than 35 years, large tumor size (T3–4), high serum NLR, high LNR, and TN phenotype were significant prognostic factors of DFS in univariate analysis. Multivariate analysis showed that young age (<35 years; p=0.009; hazard ratio [HR], 5.611; 95% confidence interval [CI], 2.340–13.462), high NLR (>3.0; p=0.020; HR, 3.934; 95% CI, 1.271–12.111), high LNR (>0.65; p=0.062; HR, 1.464; 95% CI, 0.502–4.291), and molecular phenotype (TN subtype; p=0.012; HR, 3.701; 95% CI, 1.161–12.524) were significantly associated with tumor recurrence. Tumor biologic subtype was the most significant factor of recurrence (Wald test=13.131, highest among significant factors mentioned above).
Kaplan-Meier survival analysis evaluating the prognostic effect of the molecular phenotype showed that DFS was longest in patients with the HR+HER2– subtype and shortest in those with the TN type (p=0.002). The 5-year DFS rates in patients with HR+HER2–, HR+HER2+, HR–HER2+, and TN subtypes were 82%, 63%, 58%, and 37%, respectively (Figure 2).
We divided this population into two subgroups using a 20% cutoff (low Ki-67 [<20%]/high Ki-67 [≥20%] groups). According to Ki-67 level, there was remarkable difference in DFS between these two groups (91.2% vs. 69.4%, respectively) (Figure 3).
pN3 breast cancer is known to have a poor prognosis. Several studies have reported an approximately 40% 5-year OS rate for pN3 breast cancer [41112] The present study found 5-year DFS and OS rates of 69.8% and 86.1%, respectively. This high OS rate might be due to current standard systemic management, such as taxane-based chemotherapy and endocrine therapy, which was administered to all patients as indicated. Patients with HER2+ tumors diagnosed before 2009 did not receive trastuzumab because it was not covered by insurance. Additional optimal targeted therapies, such as trastuzumab administration on the basis HER2 status, might be needed to improve clinical outcomes in patients with pN3 breast cancer. We analyzed survival in HER2+ subgroups with or without administration of trastuzumab, and we found a better DFS in the trastuzumab-treated group than in the no trastuzumab group (67.5% vs. 48.3%, p=0.041).
In the present study, age 35 years was related to very poor DFS in both univariate and multivariate analysis. Menopausal status was not related to prognosis in our analysis, but we believe that young age (35 years) might have prognostic power in pN3 breast cancer. In addition, the TN subtype is more common in the young age subgroup and is regarded to have contributed to the age effect on prognosis.
As shown in our analysis, high nodal ratio appeared to be a significant prognostic factor in DFS. Similar to previous studies, using a cutoff of 0.65, maximal surgical excision of LNs is therefore recommended in patients who present with advanced nodal status [813]. This study also found that pretreatment NLR was significantly related to prognosis. NLR is thought to reflect systemic inflammation and to be independently prognostic of survival in patients with various cancers [71415]. Similar to previous studies, we found that a high preoperative NLR was associated with poor patient prognosis.
We believe that tumor biology is the most significant factor of tumor recurrence. In our analysis, patients with HR+ HER2– tumors in this cohort lived significantly longer than did patients in the other subgroups. This favorable subgroup includes variable Ki-67 proliferation index results, and using a 20% cutoff we divided this population into two subgroups (low Ki-67/high Ki-67 groups). Interestingly, a more favorable outcome was observed in the high Ki-67 subgroup (luminal B subtype) than in the low Ki-67 subgroup (luminal A subtype). Breast cancers expressing high levels of Ki-67 are associated with worse prognosis, but are associated with a good chance of clinical response to chemotherapy [16]. Very high levels of tumor Ki-67 expression were an adverse prognostic factor and also suggested a predictive value for greater benefit from endocrine therapy in postmenopausal women [17]. High Ki-67 scoring is recognized to indicate a probable chemotherapy benefit in HR+HER2– breast cancer [18]. In the present study, other subgroups besides the HR+HER2– subgroup showed no distinctive prognosis according to Ki-67 expression levels. In our previous report, Ki-67 index predicted chemoresponsiveness only in ER– or HER2+ tumors, but its independent significance is modest in ER+HER2– tumors [19]. Similarly, the individual Ki-67 index does not seem to be a predictive marker of relative treatment efficacy and is not adequate for treatment selection for patients with HR+HER2– disease [10]. The debate on the prognostic role of Ki-67 in breast cancer is still open, although most the studies have established a relationship between Ki-67 and OS and DFS [20]. In addition, there is a lack of clarity regarding how Ki-67 measurements should influence clinical decisions and the threshold to be used. We have to point out that the Ki-67 cutoff may change according to different variables and technical differences in IHC staining. In our study, PR negativity in the HR+HER2– subgroup was seen in only 3%. The absence of PR expression is categorized as luminal B (high Ki-67 level with HR+HER2– subtype) and confers a worse prognosis [21]. However, the small number of PR negative patients in our study group might bring a better prognosis in luminal B subtype than was observed in other studies. Moreover, since trastuzumab was not administered to most patients with HER2+ breast cancers due to insurance issues, the survival of patients with HER2+ breast cancers may have been underestimated in this study.
The TN subtype constitutes 13.2% of our study population, and had a worse prognosis (5-year DFS, 37%) than did other subgroups. Studies have suggested that TNM staging should incorporate tumor marker profiles, such as biomarkers [22232425]. For example, TN phenotype status was found to be a significant prognostic indicator in these patients, and incorporating TN phenotype into breast cancer staging was not only feasible; moreover, it improved prognostic accuracy. Furthermore, intrinsic subtype was shown to have a prognostic impact in patients with stage I–III breast cancer, with prognostic discordance observed between TNM staging and biologic subtypes [23].
In conclusion, although this present study has some limitations of being retrospective and a single institution study, our findings suggest that pN3 breast cancer was found to be heterogeneous in terms of prognosis. Our data indicated that young age, high NLR, and high LNR, in addition to TN phenotype, were the independent prognostic factors for tumor recurrence, and tumor biology was the best predictor for DFS in pN3 breast cancer patients. New strategies, such as targeted approaches as well as novel combinations, should be considered for application in pN3 patients with the TN phenotype, rather than conventional treatment.
Figures and Tables
 | Figure 1Kaplan-Meier survival curve of study population (A) Disease-free survival (B) Overall survival. |
 | Figure 2Disease-free survival according to four groups of tumor biology.HR=hormone receptor; HER2=human epidermal growth factor receptor 2; TN=triple negative.
|
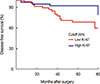 | Figure 3Subanalysis in HR+HER2– group according to Ki-67 level.HR=hormone receptor; HER2=human epidermal growth factor receptor 2.
|
Table 1
Characteristics of study population (n=220)

Table 2
Recurrence type of study population

| Recurrence type* | No. (%) |
|---|---|
| Total recurrence | 75 (34.1) |
| Locoregional only | 14 (18.7) |
| Distant metastasis only | 42 (56.0) |
| Both | 19 (25.3) |
Table 3
Factors related to disease-free survival

References
1. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002; 20:3628–3636.

2. Kuru B, Camlibel M, Dinc S, Gulcelik MA, Alagol H. Prognostic significance of axillary node and infraclavicular lymph node status after mastectomy. Eur J Surg Oncol. 2003; 29:839–844.

4. Montero AJ, Rouzier R, Lluch A, Theriault RL, Buzdar AU, Delaloge S, et al. The natural history of breast carcinoma in patients with > or=10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: a multiinstitutional retrospective study. Cancer. 2005; 104:229–235.

5. Jones SE, Moon TE, Bonadonna G, Valagussa P, Rivkin S, Buzdar A, et al. Comparison of different trials of adjuvant chemotherapy in stage II breast cancer using a natural history data base. Am J Clin Oncol. 1987; 10:387–395.

6. Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S, et al. Clinical outcome of breast cancer patients with N3a (≥10 positive lymph nodes) disease: has it changed over years? Med Oncol. 2011; 28:726–732.

7. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013; 88:218–230.

8. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009; 27:1062–1068.

9. Yonemori K, Tsuta K, Shimizu C, Hatanaka Y, Hashizume K, Ono M, et al. Immunohistochemical expression of PTEN and phosphorylated Akt are not correlated with clinical outcome in breast cancer patients treated with trastuzumab-containing neo-adjuvant chemotherapy. Med Oncol. 2009; 26:344–349.

10. Regan MM, Pagani O, Francis PA, Fleming GF, Walley BA, Kammler R, et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat. 2015; 154:275–286.

11. Duraker N, Caynak ZC, Bati B. Is there any prognostically different subgroup among patients with stage IIIC (any TN3M0) breast carcinoma? Ann Surg Oncol. 2008; 15:430–437.

12. Diab SG, Hilsenbeck SG, de Moor C, Clark GM, Osborne CK, Ravdin PM, et al. Radiation therapy and survival in breast cancer patients with 10 or more positive axillary lymph nodes treated with mastectomy. J Clin Oncol. 1998; 16:1655–1660.

13. Duraker N, Bati B, Çaynak ZC, Demir D. Lymph node ratio may be supplementary to TNM nodal classification in node-positive breast carcinoma based on the results of 2,151 patients. World J Surg. 2013; 37:1241–1248.

14. Hong J, Mao Y, Chen X, Zhu L, He J, Chen W, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016; 37:4135–4142.

15. Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016; 37:361–368.

16. Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011; 13:R22.

17. Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008; 26:5569–5575.

18. Criscitiello C, Disalvatore D, De Laurentiis M, Gelao L, Fumagalli L, Locatelli M, et al. High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast. 2014; 23:69–75.

19. Kim KI, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014; 17:40–46.

20. Kontzoglou K, Palla V, Karaolanis G, Karaiskos I, Alexiou I, Pateras I, et al. Correlation between Ki67 and breast cancer prognosis. Oncology. 2013; 84:219–225.

21. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.

22. Bagaria SP, Ray PS, Sim MS, Ye X, Shamonki JM, Cui X, et al. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014; 149:125–129.

23. Jung HA, Park YH, Kim M, Kim S, Chang WJ, Choi MK, et al. Prognostic relevance of biological subtype overrides that of TNM staging in breast cancer: discordance between stage and biology. Tumour Biol. 2015; 36:1073–1079.

24. Veronesi U, Zurrida S, Viale G, Galimberti V, Arnone P, Nolè F. Rethinking TNM: a breast cancer classification to guide to treatment and facilitate research. Breast J. 2009; 15:291–295.

25. Yi M, Mittendorf EA, Cormier JN, Buchholz TA, Bilimoria K, Sahin AA, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011; 29:4654–4661.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download