Abstract
Purpose
This study examines the effects of a rehabilitation program on quality of life (QoL), cardiopulmonary function, and fatigue in breast cancer patients. The program included aerobic exercises as well as stretching and strengthening exercises.
Methods
Breast cancer patients (n=62) who had completed chemotherapy were randomly assigned to an early exercise group (EEG; n=32) or a delayed exercise group (DEG; n=30). The EEG underwent 4 weeks of a multimodal rehabilitation program for 80 min/day, 5 times/wk for 4 weeks. The DEG completed the same program during the next 4 weeks. The European Organization for Research and Treatment of Cancer-Core Quality of Life Questionnaire (EORTC QLQ-C30), EORTC Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23), predicted maximal volume of oxygen consumption (VO2max), and fatigue severity scale (FSS) were used for assessment at baseline, and at 2, 4, 6, and 8 weeks.
Results
After 8 weeks, statistically significant differences were apparent in global health, physical, role, and emotional functions, and cancer-related symptoms such as fatigue and pain, nausea, and dyspnea on the EORTC QLQ-C30; cancer-related symptoms involving the arm and breast on the EORTC QLQ-BR23; the predicted VO2max; muscular strength; and FSS (p<0.050), according to time, between the two groups.
Every year, more than 1 million women are diagnosed with breast cancer worldwide. According to the results of research conducted by the Ministry of Health and Welfare, the incidence of breast cancer in South Korea is 15.1%. This percentage makes breast cancer the second-most frequent cancer in South Korea [1]. As opposed to the United States, where almost all patients with breast cancer are over the age of 65 years, 60% of Korean breast cancer patients are <50 years old [2]. The 5-year life expectancy of patients with breast cancer in South Korea is high at 89.5% [1].
Patients with breast cancer commonly experience symptoms of fatigue, pain, anxiety, and depression. For those who have undergone mastectomy, common sequelae of the procedure include shoulder joint limitations, weakness in the arms and hands, lymphedema, pain, and sensory problems. Adjuvant therapies, such as hormone therapy, radiation, and chemotherapy also decrease recurrence of breast cancer and mortality, but cause considerable side effects and negatively impact quality of life (QoL) [3]. Short-term side effects of radiation include fatigue and skin rash. In the long-term, radiation can cause lymphedema, cardiopulmonary toxicity, and brachial nerve palsy. Chemotherapy has toxic effects on healthy cells and tissues; its short-term negative side effects include nausea, diarrhea, headache, thrombosis, muscle pain, neuropathic problems, and fatigue. The long-term effects of chemotherapy include premature menopause, weight gain, decreased cardiac function, cognitive problems, anxiety, and depression [4].
Breast cancer and its treatment cause a general reduction in physical activity that varies in degree depending on the severity of the disease and treatment. Fatigue is the most common symptom of breast cancer and its treatment, and it tends to be the most difficult and prolonged of all symptoms [4]. To summarize, breast cancer and its treatment pose many challenges to the patient's physical, emotional, mental, and social well-being and negatively impact the patient's QoL.
Recently, it has become clear that rehabilitation involving physical activity is an important part of cancer treatment. Patients with cancer initially experience a decrease in daily activity with chemotherapy and a potential increase in weight. Cardiopulmonary function is often adversely affected, and bone mineral density often decreases with diagnosis and treatment. Exercise has been shown to be an effective intervention for improving QoL and also helps improve cardiopulmonary and physical functioning while reducing fatigue [5]. Many recommend rehabilitation that can help address both the physical and emotional contingencies of cancer, as these can cause difficulties in working; the idea is to restore function, help patients return to their occupations, and improve their QoL. Rehabilitation that specifically addresses physical and emotional functioning has been shown to be the most effective approach [6].
Aerobic and resistive exercises are effective in improving QoL and reducing the level of pain. Additionally, it can help improve the shoulder range of motion in patients with breast cancer and help alleviate fatigue. In many different studies, it has been shown that a rehabilitation program can contribute to decreasing the side effects of breast cancer [7,8,9]. Additionally, core stability exercise and massage therapy were shown to improve both physical functioning and emotional function/mood compared with a control group [7].
While the incidence rates of cancer are increasing in Korea, and rehabilitation is therefore of greater interest to these patients, most hospitals do not have specific rehabilitation programs for patients with cancer. Few studies have performed evaluations of the maximal volume of oxygen consumption (VO2max) in this patient population and applied it to core stability exercises during intervention. Given the status of current research, this study aimed to determine the effects of a 4-week stretching, aerobic, and strengthening exercise program on QoL, fatigue, and physical fitness in survivors of breast cancer. We hypothesized that a multimodal rehabilitation program would result in meaningful improvements in QoL, fatigue, and physical fitness. To test this hypothesis, we implemented a systematic multimodal rehabilitation program that would allow measurement of these parameters.
This prospective, randomized, controlled trial with a crossover design was conducted at the Rehabilitation Center at Asan Medical Center. Ethics approval was granted by the Health Institutional Review Board at Seoul National University (19-2013-04-24), and written informed consent was obtained from all participants. A total of 212 participants signed informed consent forms and were randomized to the study groups. After enrollment and randomization, 150 participants discontinued, leaving 62 participants who completed the study. All participants were evaluated at baseline and at 2, 4, 6, and 8 weeks. Overall, 150 participants failed to return for evaluation visits when the rehabilitation program was not performed in the hospital (Figure 1). The study had two arms: the early exercise group (EEG; n=32) completed the exercise program from baseline to 4 weeks, whereas the delayed exercise group (DEG; n=30) completed the exercise program from 4 to 8 weeks.
The recruited participants were patients with stage 0-3 breast cancer who had been prescribed multimodal rehabilitation after being evaluated by a physiatrist. All the patients underwent radiation therapy. Some of the participants had lymphedema, but they were able to maintain the status after complex decongestive physical therapy. Before testing, the patients were randomized by investigators into either the EEG or the DEG at a 1:1 ratio using a computer-generated allocation sequence. Women were excluded if they had evidence of recurrent disease or had other musculoskeletal involvement such as low back pain, disc problems, osteoarthritis, rheumatoid arthritis, or shoulder problems.
QoL was evaluated based on the European Organization for Research and Treatment of Cancer-Core Quality of Life Questionnaire (EORTC QLQ-C30) (version 3) and the Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23). The EORTC QLQ-C30 is a self-administered questionnaire consisting of 30 items that incorporates five functional scales (physical, functional, cognitive, emotional, and social performance); three symptom scales (fatigue, pain, and nausea and vomiting), and the scales of QoL and overall health status. In conjunction with the EORTC QLQ-C30, the Breast Specific Module, BR23, was applied. The BR23 assesses specific aspects of breast cancer and comprises 23 items with two scales: functional and symptomatic. These questionnaires have been validated and crossculturally tested in various cancer populations [10].
Cardiorespiratory function was measured using the cycle test (Ergoline 200k; Ergoline GmbH, Bitz, Germany). Patients commenced cycling at 20 W; this workload was increased by 25 W every minute. The test was completed when patients reached 85% of their estimated maximal heart rate. The cardiorespiratory test score was assessed as the power output that coincided with the 85% maximal heart rate [11].
The Fatigue Severity Scale (FSS) consists of nine questions that are responded to using a Likert scale ranging from 1 to 7, with lower scores meaning "disagreement" (greater disagreement with lower scores), and higher scores meaning "agreement" in the same fashion. A score ≥36 is regarded as severe chronic fatigue, and a score <36 indicates mild fatigue. The reliability and validity of this questionnaire has been determined before; interreliability has been shown to be at a high level [12].
Maximal isometric strength was assessed in four muscle groups bilaterally using a hand-held digital dynamometer (Power Track II Commander; JTech Medical, Salt Lake City, USA). The muscles assessed included the elbow flexors, hip flexors, hip abductors, hip extensors, knee extensors, and knee flexors. Muscular groups were tested in the middle of the joint range [13]. The system was regulated so that it recorded the maximum contraction. For every group of muscles, a duration of 5 seconds was used to measure the maximal isometric contraction. The average of three contraction trials was recorded as the final number, and 2 minutes of rest was given after every contraction to avoid a decline in strength across trials due to fatigue.
Participants were asked to attend the rehabilitation center 5 times a week for 4 weeks. The sessions were supervised by one physical therapist. The warm-up period was conducted for 10 minutes and consisted of six different upper stretching exercises and five different lower stretching exercises. For strengthening exercises of the extremities, participants performed two sets of 8-12 repetitions using the TheraBand (Hygenic Corp., Akron, USA) at 60%-80% of 1 repetition maximum and progressed to a heavier intensity. For strengthening exercises of the axial muscles, the core stability exercises were performed using a ball and consisted of nine different exercises, and the participants performed 5-10 repetitions. Aerobic exercise was performed for 40 minutes at 40%-75% of VO2max (Table 1) [14]. The intensity of the aerobic and strengthening exercises was set based on the guidelines or older adults, provided by the American College of Sports Medicine (ACSM). All lymphedema patients wore compression stockings during the exercises.
The multimodal rehabilitation program consisted of strengthening exercises using the TheraBand and stretching exercises using the T-bar; these exercises were particularly focused on the pectoralis muscle. Core stability exercises were also used, with a medicine ball used for improving the abdominal and lumbo-pelvic muscle strength (Supplementary Table 1). Finally, an aerobic exercise program component that used cycle and arm ergometers and a stepper machine was included.
During weeks 1-4, the EEG participated in the rehabilitation program, and the DEG was asked not to attend the program during this period. During weeks 4-8, the DEG was provided with the same rehabilitation program. The EEG was not given any rehabilitation program to follow during weeks 4-8. However, we encouraged both groups to exercise at home. All participants were evaluated for lymphedema, cardiorespiratory function, muscle strength of elbow flexors, hip flexors, hip extensors, and knee extensors, and responded to the fatigue and QoL questionnaires at baseline. A physical therapist assessed the active flexion, abduction, internal rotation, and external rotation range of motion of the shoulder at baseline and postexercise. Additionally, at weeks 2, 4, 6, and 8, the participants answered the questions on the FSS and EORTC survey, and were again evaluated for cardiorespiratory function. Muscle strength was assessed preintervention and postintervention.
Statistical analysis was performed using SPSS version 12.0 software (SPSS Inc., Chicago, USA). Baseline descriptive statistics were compared using independent t-tests for continuous data and chi-square analysis for categorical data. The primary analysis employed a 2 (group) by 5 (time) repeated measures analysis of variance (RM-ANOVA) to test for interactions between "time" and "group" with respect to QoL, fatigue, and cardiorespiratory fitness. Paired sample t-tests were used to assess changes in fitness over the 4-week intervention period in both the EEG and the DEG, while an independent sample ttest was used to compare the fitness value between the groups at 4 weeks. All tests were two-tailed with statistical significance set at an α level of 0.05.
The average age of the participants was 47.1±8.5 years in the EEG, and 48.3±8.2 years in the DEG; the percentage of patients with a body mass index >25 kg/m2 was 46.8% in the EEG and 33.3% in the DEG. No significant differences existed between the EEG and the DEG with respect to occupational status, average exercise frequency, education status, marriage, economic status, lymphedema, chemotherapy, hormone therapy, cancer stage, and surgery method (Table 2).
In the baseline evaluation, there were no differences in the QoL, functional aspects such as physical and emotional performance, and symptomatic aspects such as vomiting, fatigue, pain, and dyspnea between the EEG and the DEG groups. Furthermore, statistical differences were not seen in the arm and affected breast symptoms on the EORTC QLQ BR23, in the FSS, or in cardiorespiratory function and muscle strength (Tables 3, 4).
The elbow flexor, hip flexor, hip extensor, and knee extensor muscle strength displayed statistically significant improvements after the rehabilitation program in the EEG and DEG (Table 4).
After 8 weeks, statistically significant differences were apparent in global health, physical functioning, role functioning, emotional functioning, and cancer-related symptoms such as fatigue and pain, nausea and dyspnea on the EORTC QLQC30, and arm and breast symptoms on the EORTC QLQBR23. Other significant differences included VO2max, and FSS (p<0.05) according to time between the two groups (Table 5). In Figure 2, RM-ANOVA revealed a significant time-by-group interaction for QoL. Follow-up paired t-tests revealed that the EEG demonstrated a significant improvement in QoL from baseline to week 4, and the DEG demonstrated a significant QoL improvement from week 4 to week 8 (Figure 2). RM-ANOVA revealed a significant time-by-group interaction for cardiorespiratory function. Follow-up paired t-tests on the interaction effects revealed that the EEG demonstrated a significant increase from baseline to week 4; conversely, scores for the DEG decreased from baseline to week 4 but demonstrated a borderline significant increase from week 4 to week 8 (Figure 3). RM-ANOVA also revealed a significant time by group interaction for FSS (Figure 4). Follow-up paired t-tests on the interaction effects revealed that the EEG demonstrated a significant decrease from baseline to week 4. Conversely, scores for the DEG increased fatigue from baseline to week 4 but demonstrated a significant decrease from week 4 to week 8 (Figure 4).
At 4 weeks, as previously noted in the introduction to this section, statistically significant differences were seen in global health score (p=0.001), physical functioning (p=0.013), emotional functioning (p=0.001), and cancer-related symptoms such as fatigue (p=0.001) and pain (p=0.001); nausea (p=0.001) on the EORTC QLQ-C30; cancer-related symptoms such as arm and breast symptoms (p=0.001) on the EORTC QLQ-BR23; and in the predicted VO2max (p=0.005) and FSS (p=0.001).
The results isolate the differences from baseline to 4 weeks for the statistically significant differences in the EEG (compared to the nonsignificant differences in the DEG) in global health, physical function, cancer-related symptoms enumerated earlier, and FSS. Cardiorespiratory function actually showed a significant decrease in DEG (p<0.050).
The findings for week 4 to week 8 revealed that the statistically significant improvements in global health, physical function (in the EORTC QLQ C30), fatigue, and cancer-related symptoms (such as symptoms in the arm and in the breast) showed durable improvement, signifying that the effects of rehabilitation are prolonged.
The purpose of the present study was to examine the effect of a 4-week exercise program consisting of various types of exercise (stretching, aerobic, and strengthening) on QoL, fatigue, and physical fitness in breast cancer survivors. We also implemented a systematic rehabilitation program in this study.
Based on the scientific data, participation in rehabilitation programs, including aerobic and strength training, should be recommended for breast cancer patients [15]. Aerobic, stretching, and strengthening exercises have already been proven to be effective exercises in breast cancer patients; however, in this study, we modified the equipment for easier use, and combined these exercises with other types. Thus, we included aerobic and strengthening exercises with a cycle and arm ergometer and a stepper machine, and other strengthening exercises, which included TheraBand and core stability exercises. Although none of the participants had shoulder involvement at baseline, it is possible that the shoulder and arm problems could emerge during radiotherapy. Thus, our program was more focused on the prevention of shoulder problems with the use of T-bar shoulder exercises. In our results, full shoulder range of motion was maintained at baseline and postintervention. On the other hand, core stability exercises are generally used to improve lumbo-pelvic control. These exercises improve the individual's ability to activate proximal muscles, providing interactive moments that would allow efficient distal muscle function. Additionally, gym ball exercises also improve the patient's motivation for exercise and have a positive effect on dynamic postural control, appropriate muscle balance, and joint function [16]. Exercise has been investigated as a means to reduce cancer treatment-induced bone loss at the spine and hip in breast cancer patients [17]. The purposes of core stability exercises in this study were to prevent misalignment and maintain axial muscle strength. We chose the multimodal rehabilitation programs in this study for prevention of physical deterioration and improvement of QoL. These exercise materials have been made simple for the participants to use, not only in the hospital and gym, but in their own homes as well.
The rehabilitation program resulted in increased physical and emotional functioning in the study population. Additionally, the EEG showed significant improvement in physical functioning (QoL measurement) from week 4 to week 8. Some of the parameters that did not show statistically significant effects with this rehabilitation program were cognition, social functioning, insomnia, appetite loss, constipation, and diarrhea.
This study will become part of the current literature that covers the parameters of functioning and well-being that are impacted (or not impacted) by a planned physical therapy program in patients with cancer and other chronic diseases. A previous study showed that supervised exercise improves physical and psychological functioning more efficiently than self-exercise [18]. Another study demonstrated that exercise programs with physical therapy clinic are more effective than home exercise programs [19]. In the absence of exercise, patients with chronic disease including cancer have greater decreases in energy from lethargy and fatigue, compromised physical functioning, weakness of muscles, and many social and cognitive challenges (depression, cognitive deficiency, and loneliness). This study may facilitate prescription of our program by physicians for hospitalized patients.
Ultimately, improving QoL is the main purpose of implementing physical rehabilitation programs in patients with cancer. A previous study reported that most studies conducted on exercise intervention for cancer have aimed at improving the patient's QoL [20]. Exercise and physical activity create a sense of control and self-confidence in patients by reducing stress and anxiety, increasing acceptance of the illness, and improving the ability to concentrate [9,21]. Similar to our results, increasing QoL and positive emotions/feelings, decreasing stress, and improving physical function have been the advantages of certain other physical rehabilitation programs [20]. According to a previous study, 12 weeks of resistive and aerobic exercise had a positive effect on QoL in patients with breast cancer, as seen by the use of Fact-B, a tool to measure QoL [9]. However, the findings have not been uniform with respect to the correlation between physical exercise and improvement in patient functioning with chronic disease. One study showed that aerobic exercise did not have an effect on self-confidence or QoL in patients with breast cancer. Another previous study, which reported that patients have a decreasing QoL with decreases in physical functioning, sexual functioning, and role functioning with chemotherapy, did not examine the potential benefits of rehabilitation [22,23].
Rehabilitation also reduced pain and fatigue and improved symptoms especially in the breast and arms in patients recovering from cancer. The interventions in this study were more focused on upper-arm exercises with the T-bar and gym ball, which is different from other similar studies. Therefore, our results were significantly different for arm and breast symptoms, which separates this study from previous studies. Some of the participants had lymphedema. These patients showed improved breast symptoms after exercise based on the EORTC BR-23 (EEG, 43.5±32.2 to 22.1±16.5, p=0.060; DEG, 48.3±24.2 to 6.6±7.8, p=0.001). Patients with arm symptoms also showed a tendency for improvement after the intervention (EEG, 26.8±19.6 to 16.2±7.7, p=0.133; DEG, 38.5±30.1 to 10.5±12.1, p=0.023). A previous study showed that upper body resistance exercise with either high or low loads does not increase the extent of swelling or severity of symptoms in breast cancer-related lymphedema patients [24]. Indeed, moderate-to-high intensity resistance exercise significantly improved muscle strength, muscle endurance, and QoL in women with breast cancer-related lymphedema. Another study has also demonstrated that a 6-month exercise program that includes weight training improves strength without increasing lymphedema in women after breast cancer treatment that included axillary clearance [25]. Most lymphedema patients do not use their affected arm freely because they believe that using that arm too much is one of the causes of the lymphedema. Our findings showed that exercise had a positive effect on breast-specific symptoms. Other studies with similar results exist. One study revealed that patients showed decreased fatigue after a 12-week exercise program [9]. Other studies reported that pain is decreased after an exercise program in patients with breast cancer [8,26]. This study finally showed the correlation between QoL and fatigue, which is that fatigue is the most reliable predictor of QoL. Conversely, improving physical function through exercise can reduce fatigue, thereby improving QoL [26].
In our study, the patients showed improved cardiorespiratory fitness, which is in agreement with the findings of a previous study [27]; here, patients with breast cancer (diagnosed through mastectomy) participated in a therapy program that involved dancing and aerobic movement for a period of 6 weeks, and a significant difference was found in cardiorespiratory fitness following this program.
Measuring improvement in VO2max using a cycle has been validated [28]. The cycle test is a very comfortable and safe method, and therefore is appropriate for measuring cardiorespiratory function in patients with cancer. In our study, after 8 weeks, the participants in the EEG showed significant improvement in VO2max. It is also important to note that aerobic exercise over 8 weeks is recommended by the ACSM [29].
With regard to the symptom of fatigue, a significant improvement was noted, as measured by the FSS. We evaluated fatigue not only using the EORTC scales but also the FSS. The National Comprehensive Cancer Network defines cancer-related fatigue as a distressing, persistent, subjective sense of physical, emotional, and cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with regular functioning. Partridge et al. [4] reported that fatigue is the most distressing symptom of cancer and effect of treatment, and is especially prolonged in patients with after chemotherapy. Generally, cancer-related fatigue lasts 1 year after cancer treatment, and greater degrees of fatigue has a more deleterious effect on the QoL. Similar to our study, in a previous study, it was found that patients receiving radiotherapy experience less fatigue, particularly after engaging in aerobic exercises [30]. Exercise in the form of walking at a comfortable level of speed and exertion also has a positive effect on fatigue. Additionally, a recent systematic review showed that exercise, including aerobics, stretching, and strengthening, has a positive effect on physical function and interferes less with daily activities [5]. However, the reason for this is unclear. One study suggests that improvement in physical functioning with the help of exercise and increasing muscle power might improve patients' efforts to overcome fatigue following radiotherapy [30]. Patients suffer from muscular deconditioning and a loss of performance if they are inactive for prolonged periods due to the fatigue. Consequently, they are more eager to engage only in normal daily activities. Throughout the exercise routines performed in the course of this study, muscular strength showed the most improvement. We think that this was one of the factors that contributed to the reduction in fatigue. Regarding the core stability exercises in our program, previously, studies have been conducted to determine the effect of these types of exercises (specifically, core stability exercises, and massage therapy) on fatigue and mood. A significant difference was found in the experimental cohort in a previous study [7].
This study was conducted with a small sample size. Although a total of 212 patients completed the rehabilitation program, 150 of them failed to complete the evaluation process. The cause of the high dropout rate was that we excluded even the results with only one incomplete evaluation. Many participants lived at long distances from the hospital and found it difficult to return just for the evaluation. Moreover, the participants were not provided monetary assistance for transportation, which may have been another reason for not reporting to the hospital for evaluation. We also did not measure the changes in circumference in lymphedema patients: This study did not consider psychological or physical changes such as pectoral muscle length and shoulder function during radiotherapy. In addition, we do not know the effect of each exercise program because our program included various exercises, and the exercise period was short. However, the 4-week program may be feasible for implementation in clinical settings.
This study had a multimodal, high-volume approach. It provides evidence for the effectiveness of a multimodal exercise program in improving QoL, reducing fatigue, and improving cardiorespiratory fitness in patients with breast cancer.
Figures and Tables
 | Figure 2Quality of life score from baseline to week 8 by group assignment (n=62).EEG=early exercise group; DEG=delayed exercise group; CI=confidence interval.
|
 | Figure 3Cardiorespiratory function from baseline to week 8 by group assignment (n=62).EEG=early exercise group; DEG=delayed exercise group; CI=confidence interval.
|
 | Figure 4Fatigue severity scores from baseline to week 8 by group assignment (n=62).EEG=early exercise group; DEG=delayed exercise group; CI=confidence interval.
|
Table 1
Physical therapy program

Table 2
Baseline demographic and medical history variables of study participants

Table 3
Comparison of the mean values for the dimensions of quality of life and cardiorespiratory fitness and fatigue in baseline between the EEG and DEG groups
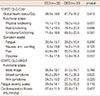
Table 4
Changes of muscular strength in preintervention and postintervention

Table 5
Change in patient-rated outcomes over the 8-week study

Data are presented as mean±SD.
EEG=early exercise group; DEG=delayed exercise group; EORTC=The European Organization for Research and Treatment of Cancer; QoL=quality of life.
*Time×group p-value=time by group interaction; †Quality of Life Questionnaire-Cancer; ‡Quality of Life Questionnaire-Breast.
References
1. National cancer statistics. Ministry of Health and Welfare: 2014. Accessed June 13th, 2014. http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040101000000.
2. Sant M, Allemani C, Berrino F, Coleman MP, Aareleid T, Chaplain G, et al. Breast carcinoma survival in Europe and the United States. Cancer. 2004; 100:715–722.

3. Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2006; (4):CD005001.

4. Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001; (30):135–142.

5. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006; 175:34–41.

6. McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. J Clin Oncol. 2003; 21:463–466.

7. Cantarero-Villanueva I, Fernández-Lao C, Del Moral-Avila R, Fernández-de-Las-Peñas C, Feriche-Fernández-Castanys MB, Arroyo-Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evid Based Complement Alternat Med. 2012; 2012:620619.

8. Hwang JH, Chang HJ, Shim YH, Park WH, Park W, Huh SJ, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008; 49:443–450.

9. Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008; 108:279–288.

10. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13:863–868.

11. Pollock ML, Wilmore JH, Fox SM. Health and Fitness through Physical Activity. New York: John Wiley & Sons;1978.
12. Taylor RR, Jason LA, Torres A. Fatigue rating scales: an empirical comparison. Psychol Med. 2000; 30:849–856.

13. Ford-Smith CD, Wyman JF, Elswick RK Jr, Fernandez T. Reliability of stationary dynamometer muscle strength testing in community-dwelling older adults. Arch Phys Med Rehabil. 2001; 82:1128–1132.

14. Kisner C, Colby LA. Therapeutic Exercise: Foundations and Techniques. 4th ed. Philadelphia: F.A. Davis;2002.
15. Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: an overview. World J Clin Oncol. 2014; 5:406–411.

16. Behm DG, Anderson K, Curnew RS. Muscle force and activation under stable and unstable conditions. J Strength Cond Res. 2002; 16:416–422.

17. Valachis A, Polyzos NP, Georgoulias V, Mavroudis D, Mauri D. Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: a meta-analysis. Gynecol Oncol. 2010; 117:139–145.

18. Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007; 334:517.

19. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001; 10:19–28.

20. Oldervoll LM, Rø M, Zwart JA, Svebak S. Comparison of two physical exercise programs for the early intervention of pain in the neck, shoulders and lower back in female hospital staff. J Rehabil Med. 2001; 33:156–161.

21. Blanchard CM, Stein KD, Baker F, Dent MF, Denniston MM, Courneya KS, et al. Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychol Health. 2004; 19:1–13.

22. Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998; 25:107–113.
23. So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M, et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009; 36:E205–E214.

24. Cormie P, Galvão DA, Spry N, Newton RU. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther. 2013; 12:423–432.

25. Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006; 24:2765–2772.

26. Lee EH, Moon S, Song Y, Chun M. Relationships of lymphedema, the shoulder range of motion, fatigue and social support to the health related quality of life in patients with breast cancer. J Breast Cancer. 2010; 13:212–218.

27. Crowley S. The effect of a structured exercise program on fatigue, strength, endurance, physical self-efficacy, and functional wellness in women with early stage breast cancer. Virginia Henderson International Nursing e-Repository;2004. Accessed September 16th, 2014. http://hdl.handle.net/10755/165449.
28. Kang DM, Park YK, Lee YH, Sul JG. Validity on submaximal load tests using cycle ergometer in evaluation of maximum oxygen consumption volume. J Korean Soc Occup Environ Hyg. 2006; 16:145–151.
29. Karageanes SJ. Principles of Manual Sports Medicine. Philadelphia: Lippincott Williams & Wilkins;2005.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download