Abstract
Purpose
The aim of this study was to investigate whether the observed changes over time in the survival rates vary according to the intrinsic subtypes of breast cancer diagnosed.
Methods
Data from 46,320 breast cancer patients in the Korean Breast Cancer Registry who underwent surgery between 1999 and 2006 were reviewed. Among them, results from 25,887 patients with available data about the status of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) were analyzed. Patients were classified into two cohorts according to the year in which they underwent surgery: 1999-2002 and 2003-2006.
Results
The patients treated in the latter time period showed significantly better overall survival (OS) compared with those in the former period when adjusted for follow-up duration. The proportion of hormone receptor+/HER2-subtype and stage I breast cancer were significantly higher in the latter period (47.4% vs. 54.6%, p<0.001; 31.0% vs. 39.6%, p<0.001, respectively). Improvement in OS between the former and latter periods was seen in all subtypes of breast cancer, including triple-negative cancers (all p-values <0.001 in univariate and multivariate analyses).
The Korean Breast Cancer Society (KBCS) publishes biennial reports on the characteristics of breast cancer in the country, along with any evolving trends [1,2,3,4]. Data from the nationwide breast cancer registry program run by the Korean Breast Cancer Registry (KBCR) show that the overall survival (OS) of Korean women with breast cancer has been improving over time [5]. However, there is limited data to explain this increasing breast cancer survival in Korea.
Several hypotheses exist to explain this improved survival in breast cancer patients over time. These include improved early detection of breast cancer, increased rate of nonaggressive biologic phenotype breast cancer, and the use of novel adjuvant hormonal or target therapy agents, such as aromatase inhibitors (AIs) and trastuzumab.
Genomic studies have established that breast cancer can be divided into four major intrinsic subtypes (hormone receptor [HR]+/human epidermal growth factor receptor 2 [HER2]-, HR+/HER2+, HR-/HER2+, and HR-/HER2-) that differ in terms of incidence, survival, and response to therapy [6,7]. Survival analysis according to these subtypes may provide some information in terms of the various hypotheses regarding the improved survival.
In this study, we have attempted to identify the clinicopathological and treatment factors that have exerted considerable influence upon the observed improvement in breast cancer survival in Korea.
The KBCR is a database that has been prospectively maintained by the KBCS since 1996 [8]. Nationwide, 102 general hospitals with at least 400 beds, including 41 university hospitals and 61 surgical training hospitals, have voluntarily participated in this program. In 2001, the Online Korean Breast Cancer Registration Program was launched, allowing physicians from each participating hospital could to input the data into the web-based database themselves. Retrospective collection of data from as far back as 1992 was allowed when a participating hospital had a breast cancer database originating before 1996. Essential items for registration were the patient's unique Korean resident's registration number as an identifier; the patient's sex and age; the surgical method used; and the cancer stage based on the American Joint Committee on Cancer classification. In 2004, this registry was estimated to include more than 50% of all newly diagnosed breast cancer patients in Korea.
Patient survival data, including dates and causes of death, were obtained from the Korea Central Cancer Registry, Ministry of Health and Welfare, Korea. The Korean Central Cancer Registry is linked to the Korean National Statistical Office, which maintains complete death statistics recorded by unique identification numbers assigned to all Korean residents [9]. The KBCR data do not include the type or date of tumor recurrence because the Korean Central Cancer Registry provides mortality data only.
The KBCR data do not provide the patient's name and unique Korean resident registration number in order to protect the privacy of each patient.
From the database, we identified 46,320 patients who underwent breast cancer surgery between January 1999 and December 2006. Among them, 2,335 patients with pure carcinoma in situ were excluded. Patients for whom immunohistochemistry (IHC) results for estrogen receptor (ER), progesterone receptor (PR), or HER2 status were not available, as well as patients who received neoadjuvant chemotherapy were also excluded. In addition, we excluded 6,125 patients who had a 2+ result for HER2 in IHC staining (Figure 1). The HER2 status in these patients should be analyzed by using fluorescence in situ hybridization (FISH); however, because we were not able to obtain FISH data in cases of equivocal IHC results, these patients were excluded. Patients who had a 0, 1+ result for HER2 in IHC staining was considered HER2-, 3+ result for that was considered HER2+.
Patients were divided into two cohorts according to the year they received surgery: Cohort I (1999-2002) and Cohort II (2003-2006). In 2003, the use of the AIs was approved by the Korean Food and Drug Administration (FDA) for treatment of early breast cancer. To adjust for the effect of the duration of follow-up between the two cohorts, we took different follow-up end points for the study such as the last date of December 2005 for Cohort I and the last day of December 2009 for Cohort II.
The study population was categorized into four breast cancer subtypes according to the HR, and HER2 status of the tumors: HR-/HER2- (ER-negative, PR-negative, HER2-negative), HR-/HER2+ (ER-negative, PR-negative, HER2-positive), HR+/HER2+ (ER-positive and/or PR-positive, HER2-positive), and HR+/HER2- (ER-positive and/or PR-positive, HER2-negative).
The correlation between the two cohorts according to the date of surgery and clinicopathological parameters was analyzed by using a chi-square test for trends. Overall survival (OS) was defined as the time from the date of surgery to the date of death or last follow-up.
Relative survival was defined as the observed survival rate divided by the expected survival rate. The observed survival rate was obtained from patient data by using the life-table method. Data from the general population matched for age, sex, and year of diagnosis were used to calculate the expected survival rate. Advice regarding estimation of relative survival was received from the Seoul National University Hospital Medical Research Collaborating Center.
The Kaplan-Meier method was used to estimate the survival outcomes of all patients by date of surgery and cancer subtype. Groups were compared by using log-rank statistics. Cox proportional hazards models were used for both univariable and multivariable analysis.
The hazard ratios (95% confidence interval [CI]) and p-values are reported. A p-value of <0.05 was used to signify statistical significance. We conducted our analyses by using the SPSS statistical software version 19.0 (SPSS Inc., Chicago, USA).
A total of 25,887 patients with invasive breast cancer with available IHC results were included in the final analysis. Patient characteristics by date of surgery are listed in Table 1. The median age of the patients at diagnosis was 47 years for both cohorts. The median duration of follow-up was 55 months for Cohort I and 48 months for Cohort II.
Cohort II had a higher proportion of older patients (>50 years), as well as patients with smaller tumor size (≤2 cm), negative node involvement, favorable histological grade (G1 or 2), negative lymphovascular invasion, positive HR status, and negative HER2 status.
Figure 2 shows the increase of HR+/HER2- subtype breast cancer. There was a higher proportion of HR+/HER2- subtype and stage I breast cancer in Cohort II as compared with Cohort I (HR+/HER2-, 54.6% vs. 47.4%, p<0.001; stage I, 39.6% vs. 31.0%, p<0.001).
Five-year OS was 85.6% for Cohort I and 93.0% for Cohort II (p<0.001). Table 2 lists the results of univariate and multivariate Cox regression analyses for clinicopathological factors influencing survival. Factors favorably affecting long-term OS rates were age <50 years, small tumor size, lymph node negativity, HR-positive, HER2-negative, low histological grade, and negative lymphovascular invasion.
The OS rate was superior in Cohort II compared to Cohort I for all subtypes (Figure 3). The 5-year breast cancer-specific survival rate was also superior in Cohort II compared to in Cohort I in each subtype: 94.7% vs. 98.0% in HR+/HER2-; 89.7% vs. 96.2% in HR+/HER2+; 82.9% vs. 94.6% in HR-/HER2+; and 87.0% vs. 94.5% in HR-/HER2-, respectively.
We also performed multivariate analysis to explore the associated prognostic factors of breast cancer according to its subtypes (Table 3). The date of surgery (Cohort II vs. Cohort I) was significantly associated with survival in all subtypes after adjustment for other prognostic factors (HR+/HER2-: hazard ratio, 0.467, 95% CI, 0.373-0.585, p<0.001; HR+/HER2+: hazard ratio, 0.458, 95% CI, 0.323-0.633, p<0.001; HR-/HER2+: hazard ratio, 0.449, 95% CI, 0.390-0.640, p<0.001; HR-/HER2-: hazard ratio, 0.582, 95% CI, 0.481-0.738, p<0.001).
We analyzed the relative survival according to the stage. In Cohort II, especially in the advanced stage, most subtypes displayed better survival than that of Cohort I. However, the HER2 subtype showed similar relative survival between Cohort I and Cohort II.
In this study, we have demonstrated that the OS rate of breast cancer in Korea improved with respect to different chronological periods. We have also shown that survival improvement was seen in all molecular subtypes. In addition, we found that the patients in Cohort II were more likely to have smaller tumors, negative lymph node involvement, and favorable histological status. Interestingly, we found that the prevalence of the HR+/HER2- subtype of breast cancer was significantly increased in Cohort II.
Remarkably, there was a significant survival improvement in patients with the HR-/HER2-subtype of breast cancer. There has been little change in the treatment of the HR-/HER2- subtype of breast cancer and there has emerged neither any accepted targeted therapy nor any effective biologic agent for this type since the breast cancer community defined this disease entity in early 2000. The increase in survival might be explained by the increase in early-stage breast cancer in the HR-/HER2- subtype, and it might also be due to increasing use of aggressive chemotherapy even for early-stage HR-/HER2- subtype of breast cancer.
One recent study from the Surveillance, Epidemiology, and End Results program demonstrated a significant decline of ER-positive breast cancer in Caucasian women in recent years [10]. However, the frequency of ER-positive breast cancer significantly increased in Japanese women [11]. In our study, we also found a similar trend among Japanese women.
A pooled analysis from the Breast Cancer Association Consortium Studies demonstrated that early age at menarche (≤12 years), nulliparity, increasing age at first birth, and obesity in older women (>50 years) were more frequently associated with HR-positive breast cancer, especially HR+/HER2- tumors [12,13]. Ko et al. [1] demonstrated that the increased incidence of breast cancer in Korea might be associated with increases in these generally accepted reproductive risk factors [14,15]. Additionally, recent nationwide Korean breast cancer data have shown that increased reproductive risk factors may explain the recent increase in HR-positive tumors, especially the HR+/HER2- subtype [16].
AIs have been approved by the Korean FDA as a treatment for postmenopausal patients with HR-positive breast tumor since 2003. In our study, 178 of 4,489 patients (4.0%) in Cohort I and 1,085 of 9,602 patients (11.3%) in Cohort II were prescribed AIs as adjuvant therapy. There was no survival difference between the group that used selective ER modulator only and the group that used AIs (p=0.206) (Figure 4). It seems that increased use of AIs may not significantly contribute to the improvement in survival rates seen over time. We could not identify the effect of trastuzumab in this study since it was not approved for use in Korea until after 2006.
Large-scale, multicenter data are one of the strengths of our study, but they are also one of the limitations. Because of the large amount of missing data, we had difficulty in investing the characteristics of all patients desired in our study. All patients enrolled in the present study were breast cancer patients with result for ER, PR, and HER2 IHC result only. Thus, caution is required when our results are interpreted as representative for Korean breast cancer survival. Data on pathologic features and HR status were not standardized between institutions. Moreover, HER2 2+ patients had to be excluded unless FISH assay was performed, which could have resulted in a different proportion of subtypes.
Improvement in Korean breast cancer survival over the study period was observed in all subtypes of breast cancer, which implies that an increase in early-stage detection and less aggressive cancers contributed to this improvement.
Figures and Tables
Figure 1
Study enrollment scheme.
HER2=human epidermal growth factor receptor 2; ER=estrogen receptor; PR=progesterone receptor; IHC=immunohistochemistry; AJCC=American Joint Committee on Cancer.
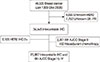
Figure 2
Chronological changes in Korean breast cancer during 1999 to 2006. The incidence of Korean breast cancer during 1999 to 2006 demonstrated by the subtype (A) and the stage (B). Proportional change of Korean breast cancer during 1999 to 2006 demonstrated by the subtype (C) and the stage (D).
HR=hormone receptor; HER2=human epidermal growth factor receptor 2.

Figure 3
Relative survival plot of Korean breast cancer according to the subtype. (A) HR+/HER2-. (B) HR+/HER2+. (C)
HR-/HER2+. (D) HR-/HER2-. HR=hormone receptor; HER2=human epidermal growth factor receptor 2.

Figure 4
Kaplan-Meier survival plot of the HR+ (ER+ and/or PR+) subtype tumor according to the use of aromatase inhibitors (AIs). There is nonsignificant survival difference between the selective estrogen receptor modulator (SERM)-only use group and AIs use group (p=0.206).
HR=hormone receptor; ER=estrogen receptor; PR=progesterone receptor; CI=confidence interval.

Table 1
Patient, tumor and treatment characteristics according to Cohort I and Cohort II

Table 2
Univariate and multivariate analysis for prognostic factors associated with overall death among Korean breast cancer patients

Table 3
Univariate and multivariate analysis for prognostic factors associated with overall death among Korean breast cancer patients according to molecular subtype

ACKNOWLEDGEMENTS
The Korean Breast Cancer Society thanks the following members who participated in the National Registry Program:
S.H. Ahn, J.W. Bae, Y.T. Bae, J.W. Baek, J.G. Bong, K.H. Cha, E.S. Chang, I.T. Chang, S.S. Chang, J.W. Cho, S.H. Cho, Y.U. Cho, B.J. Chae, J.W. Choi, K.J. Choi, M.S. Choi, S.I. Choi, S.Y. Choi, G.S. Goo, S.H. Han, W. Han, S.J. Hong, J.Y. Hwang, T.I. Hyun, Y.J. Jegal, M.G. Im, Y.G. Joh, S.Y. Jun, B.W. Jung, J. Jung, J.H. Jung, K.H. Jung, M.S. Jung, P.J. Jung, S.H. Jung, S.S. Jung, Y.H. Jung, Y.S. Jung, D.H. Kang, H.J. Kang, H.S. Kang, S.H. Kang, Y.I. Kang, Y.J. Kang, J.H. Keum, D.Y. Kim, E.K. Kim, H.A. Kim, H.J. Kim, J.G. Kim, J.H. Kim, J.S. Kim, J.S. Kim, K.C. Kim, M.S. Kim, S.C. Kim, S.H. Kim, S.I. Kim, S.J. Kim, S.K. Kim, S.W. Kim, S.W. Kim, S.Y. Kim, S.Y. Kim, T.H. Kim, Y.S. Kim, B.K. Ko, S.S. Ko, S.H. Koh, B.H. Koo, J.Y. Koo, B.S. Kwak, H.S. Kwak, C.H. Lee, C.H. Lee, D.H. Lee, D.S. Lee, E.S. Lee, G.S. Lee, H.D. Lee, H.S. Lee, J.C. Lee, J.E. Lee, J.H. Lee, J.K. Lee, J.S. Lee, J.Y. Lee, K.M. Lee, K.P. Lee, K.S. Lee, K.Y. Lee, M.H. Lee, R.A. Lee, S.C. Lee, S.J. Lee, S.K. Lee, S.Y. Lee, W. Lee, Y.H. Lee, J.W. Leu, C.H. Lim, C.W. Lim, W.S. Lim, B.I. Moon, Y.S. Nam, S.J. Nam, D.Y. Noh, W.C. Noh, S.J. Oh, S.S. Oh, W.K. Pae, I.W. Paik, N.S. Paik, B.G. Park, B.W. Park, C.H. Park, E.H. Park, H.B. Park, H.K. Park, H.Y. Park, J.H. Park, K.H. Park, M.H. Park, S.H. Park, S.J. Park, S.T. Park, S.W. Park, W.C. Park, Y.K. Park, Y.L. Park, H.S. Seo, K.H. Seo, Y.J. Seo, Y.S. Sin, B.H. Son, G.S. Son, B.J. Song, K.H. Song, Y.J. Song, Y.J. Suh, J.M. Won, D.H. Woo, D.H. Yang, J.H. Yang, K.Y. Yoo, S.Y. Yoo, H.J. Yoon, H.S. Yoon, J.H. Yoon, and S.O. Yoon.
Notes
References
1. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.

2. Korean Breast Cancer Society. Clinical characteristics of breast cancer patients in Korea in year 2000. J Korean Breast Cancer Soc. 2002; 5:217–224.
3. The Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2002 The Korean Breast Cancer Society. J Korean Breast Cancer Soc. 2004; 7:72–83.
4. Jung YS, Na KY, Kim KS, Ahn SH, Lee SJ, Park HK, et al. Nation-wide Korean breast cancer data from 2008 using the breast cancer registration program. J Breast Cancer. 2011; 14:229–236.

5. The Korean Breast Cancer Society. Survival analysis of Korean breast cancer patients diagnosed between 1993 and 2002 in Korea: a nationwide study of the cancer registry. J Breast Cancer. 2006; 9:214–229.
6. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752.

7. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.

8. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea--a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368.

9. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999.2001: first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–331.

10. Hausauer AK, Keegan TH, Chang ET, Clarke CA. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007; 9:R90.

11. Yamashita H, Iwase H, Toyama T, Takahashi S, Sugiura H, Yoshimoto N, et al. Estrogen receptor-positive breast cancer in Japanese women: trends in incidence, characteristics, and prognosis. Ann Oncol. 2011; 22:1318–1325.

12. Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011; 103:250–263.
13. Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012; 131:159–167.

14. Yoo KY, Kim Y, Park SK, Kang D. Lifestyle, genetic susceptibility and future trends of breast cancer in Korea. Asian Pac J Cancer Prev. 2006; 7:679–682.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download