Abstract
Purpose
The present study was conducted to investigate the proportion and clinical outcomes of breast cancer patients who did not receive postoperative radiotherapy (PORT) after breast-conserving surgery (BCS).
Methods
This retrospective study included all breast cancer patients received curative BCS without PORT between 2003 and 2013. In the PORT omission group, characteristics and local recurrence differences were compared between the recommended group and the refused group. To compare the local recurrence-free survival (LRFS) of the PORT omission group and the control group who received PORT, subjects were selected by using the pooled data of patients treated between 1994 and 2007.
Results
During the study period, 96 patients did not receive PORT among a total of 6,680 patients who underwent BCS. Therefore, the overall rate of PORT omission was 1.4%. Among the 96 patients, 20 were recommended for PORT omission (recommended group) and 76 refused PORT (refused group). The median follow-up period of all study participants was 19.3 months (range, 0.3-115.1 months). Patients in the recommended group were older (p=0.004), were more likely to be postmenopausal (p=0.013), and had more number of positive prognostic factors compared with the refused group. Overall, 12 cases of disease recurrence, including 11 cases of local recurrence, developed in the PORT-refused group. The LRFS of the PORT-omission group was significantly inferior to that of patients who received PORT after BCS (p<0.001). In the PORT-omission group, significant favorable prognostic factors for LRFS were having histologic grade 1 or 2 disease (p=0.023), having no axillary lymph node metastasis (p=0.039), receiving adjuvant endocrine therapy (p=0.046), and being in the recommended group (p=0.026).
Breast cancer is the second most common malignancy in women in the United States after skin cancer [1]. It is one of the primary causes of cancer-related death in women worldwide and the fifth leading cause in Korea [2,3]. Furthermore, the incidence of breast cancer is increasing with population aging, changing reproductive patterns, and increasingly active screening [1,4]. Fortunately, the treatment outcomes of breast cancer have shown considerable improvement in recent decades, primarily because of stage/subtype-specific multi-modality treatment [1,4].
Postoperative radiotherapy (PORT) is one of the essential breast cancer treatment modalities, and a positive effect of PORT after breast-conserving surgery (BCS) has clearly been demonstrated in a large long-term study [5]. Because of these proven benefits, PORT is recommended for all breast cancer patients who receive BCS [6,7]. However, some patients do not receive PORT for various reasons, such as a fear of radiation, difficulty in accessing a radiation facility, or short life expectancy. According to the Surveillance, Epidemiology, and End Results (SEER) registry, approximately 10% to 20% of patients did not receive PORT after BCS [8], and those patients had lower cancer-specific survival than patients who did receive PORT [9]. On the other hand, studies have indicated that some patients can omit PORT without an increase in their recurrence risk [10,11,12,13,14]. The clinical outcomes of PORT omission after BCS have not been studied in a Korean population.
The present study was designed to investigate the proportion and clinical outcomes of breast cancer patients who did not receive PORT after BCS at Samsung Medical Center in Korea.
The present retrospective study was conducted on all breast cancer patients who received curative BCS at Samsung Medical Center between January 2003 and June 2013. Our Institutional Review Board approved this study and waived the requirement for consent (IRB number: 2014-07-020).
The inclusion criteria were as follows: female patients who were pathologically diagnosed with invasive breast cancer, no distant metastasis (DM) detected before surgery and for at least 6 months after surgery, and curative BCS performed without subsequent adjuvant radiotherapy (RT) within 1 year. The ineligibility criteria were as follows: presence of in situ lesion(s) only, ipsilateral tumor recurrence after curative local treatment, completion of total mastectomy within 6 months after BCS, prior or simultaneous invasive malignancy except for papillary thyroid carcinoma, and/or minimum 2-year disease-free interval.
This study was based on all registered data coded as invasive breast cancer in the Samsung Medical Center Information System based on the above criteria. Initially, we searched for patients who were diagnosed with invasive breast cancer and who received resection surgery in our hospital without a record of RT in the following year. Patients who underwent a total mastectomy or completed mastectomy after BCS were excluded. For all remaining patients, we confirmed whether PORT was conducted in another institution through a careful review of medical records, and we excluded patients with confirmed definite administration of PORT. The present study was conducted on the remaining eligible patients.
The clinical, pathologic, or immunohistochemical (IHC) data were collected. According to the IHC profile, molecular subtypes were classified as follows: luminal (positive for estrogen receptor [ER] or progesterone receptor [PR], and negative for human epidermal growth factor receptor 2 [HER2]), luminal/HER2+ (positive for ER or PR and positive for HER2), HER2+/ER-PR- (negative for ER/PR and positive for HER2), and triple-negative (negative for all ER, PR, and HER2) [15].
Local recurrence (LR) was defined as tumor reappearance in the ipsilateral breast; regional recurrence (RR) as recurrence in axilla, internal mammary area, infraclavicular or supraclavicular regions; and locoregional recurrence (LRR) as either LR or RR. DM was defined as tumor recurrence outside the above locoregional areas. Biopsy or excision was performed if additional pathologic and/or IHC examination was needed for optimal systemic management or if solitary LRR was suspected; however, either clinical or radiologic examination alone was sufficient to diagnose recurrence in patients who had a poor performance status and/or multiple sites or multiple lesions.
The Fisher exact test and Mann-Whitney test were used to compare clinical, pathologic, or IHC variables between groups. The date of operation was regarded as the start of follow-up for survival analysis. Local recurrence-free survival (LRFS) was measured from the date of the operation to the date when LR developed or the final follow-up visit if LR was not detected during the follow-up period.
To compare the LRFS of the PORT-omission group and the control group who received PORT, subjects were selected from pooled data of 1,015 patients with invasive ductal carcinoma and 56 patients with invasive lobular carcinoma treated between 1994 and 2007 at our institution. Patients who received neoadjuvant chemotherapy before surgery were excluded from the control group. The pathologic stage was I in 588 cases (54.9%), II in 433 (40.4%), and III in 50 (4.7%).
The Kaplan-Meier method and log-rank test were used to calculate survival curves and compare differences between the curves, respectively. All statistical analyses were performed using the Windows version of PASW software version 21.0 (SPSS Inc., Armonk, USA), and p<0.05 was considered statistically significant.
During the study period, a total of 6,680 patients received BCS with curative intent at Samsung Medical Center. Among them, BCS was conducted as a primary treatment without RT within 1 year at our institution for 1,210 female patients with invasive breast cancer. Of these, 1,088 patients received PORT at other institutions. Therefore, 122 patients who definitely did not receive PORT remained eligible for this study. Among these patients, an uncontrolled prior malignancy was present in five patients and one patient committed suicide after surgery. During chemotherapy, recurrence was detected in 18 patients, and two patients died because of possible chemotherapy-related respiratory failure without evidence of tumor recurrence. The present study was conducted with the remaining 96 patients (Figure 1). The median follow-up period of those patients was 17.2 months (range, 0.3-115.1 months).
Omission of PORT was recommended by the physician for patients with stage I disease and an age more than 70 years (n=13), and it was optionally recommended for those with small tumors with a wide margin (stage T1mi, surgical margin ≥5 mm, ductal carcinoma in situ smaller than 1 cm; n=7). These 20 patients formed the recommended group. Seventy-six patients refused PORT, even though their doctor advised PORT after BCS (refused group).
The comparison of patient characteristics between the two groups is shown in Table 1. As expected, the recommended group had an advanced age (p=0.004), a higher percentage of postmenopausal patients (p=0.013), no axillary lymph node (LN) metastasis (p<0.001), and a higher proportion of nuclear grade 3 disease (p=0.009). Additionally, in the molecular subtype classification, the recommended group showed a higher proportion of the luminal type, and lower proportions of luminal/HER2+ and triple-negative types than the refused group (p=0.041).
Because of the older age of the patients and the small tumor size, adjuvant chemotherapy was not used in the recommended group, in contrast to the refused group (p=0.003). Endocrine therapy, however, was more frequently used in the recommended group than in the refused group (p=0.001), although the positivity status of hormone receptors was not significantly different between the groups.
The proportion of PORT omission after BCS from the relevant study period was calculated based on our institutional breast cancer database and was classified by surgery type. The overall rate of PORT omission between January 2003 and June 2013 was 1.4% (96 of 6,680 BCS cases). The annual proportion of PORT omission for all patients and separately for the two groups is shown in Figure 2. The data showed a slight increase in the PORT omission rate to approximately 1.5% after 2006, when physicians started to recommend PORT omission in certain cases.
LR with or without RR was the most frequent failure pattern in the study patients. Overall, 12 recurrences, developed during the follow-up period, and they were all in the refused group. Among the 12, five were solitary LR only and five were LRR. The other two patients showed DM, one of which was combined with LRR. The median time to recurrence was 9.8 months (range, 6.4-50.2 months).
Nine of the patients who showed LRR were recommended for salvage surgery with adjuvant RT and chemotherapy; the remaining patient showed recurrence on the breast, axilla, and internal mammary LN and underwent salvage RT followed by chemotherapy. Only four patients received the recommended treatment; three of these had no evidence of recurrence during the median follow-up of 18.9 months (range, 17.5-43.0 months), whereas the other patient showed DM after 20 months. Two patients received surgical resection only and refused to undergo RT and chemotherapy; one showed recurrence after 8.9 months, and the other was lost to follow-up 1 month after surgery. The remaining four patients who showed LRR refused to get any treatment and were lost to follow-up.
The overall LRFS rate of the PORT omission group was 80.0% at 3 years and 75.6% at 5 years; this result was significantly inferior to the result of a recent analysis of 1,071 patients who received PORT after BCS at our institution (p<0.001) (Figure 3).
Most cases of LR developed within 3 years after surgery. Possible prognostic factors related to LRFS are shown in Table 2. Significant favorable prognostic factors were histologic grade 1 or 2 (p=0.023), no axillary LN metastasis (p=0.039), adjuvant endocrine therapy (p=0.046), and the recommended group (p=0.026). Figure 4 shows LRFS curves according to these significant factors.
A proportion of patients with breast cancer do not receive PORT after BCS [16], even though its benefit has been repeatedly confirmed by multiple meta-analyses of large, well-designed, randomized, controlled trials [17,18]. PORT after BCS can reduce both LR and DM, thus improving overall survival [5]. Therefore, guidelines recommend PORT as an integral component of breast cancer management after BCS [5,6]. Nonetheless, 10% to 20% of breast cancer patients in the United States do not receive PORT after BCS [8,16].
Several studies have shown that the effect of PORT after BCS on disease recurrence and overall survival with endocrine therapy is minimal in older patients (aged 70 or more) with stage I disease [13,19]. Based on these results, National Comprehensive Cancer Network guidelines now allow PORT omission after BCS in these patients [6].
Breast cancer is one of the most important health-related issues for Korean women [3,4]. Korea has the most rapidly increasing incidence of breast cancer among the nations of the Organization for Economic Co-operation and Development. There is a slight difference in the breast cancer distribution according to age, and patients aged 70 years or older account for only 5% to 6% of all cases of breast cancer in Korea according to data from 2006 to 2010 [4]. In contrast, more than 40% of breast cancer cases developed in women aged 65 years or older in the United States [1]. Because of these unique characteristics, it is necessary to investigate the proportion and clinical outcomes of PORT omission in Korean breast cancer patients.
Although a large number of patients did not receive PORT after BCS in our institution, more than 90% of these received PORT in regional hospitals. Overall, approximately 1.5% of all patients who received BCS did not receive PORT. This rate is extremely low compared with those reported in other countries [8,9,16,20]. The support of the National Health Insurance Service might be an important reason for this high rate of optimal management. Adequate evaluation of breast cancer by the Health Insurance Review and Assessment Service might be another reason for the high compliance with standard treatments.
The results of large randomized trials on PORT omission in a low-risk older population [13,19] predict favorable clinical outcomes for the patients who were recommended PORT omission in the present study, although the small number of cases and short follow-up period preclude a concrete conclusion. Watchful waiting might be an option for these patients. However, optimal indications based on molecular subtype and/or genomic analyses and careful follow-up are recommended [21,22,23], because, even in this subset of patients, the rate of LR is higher in the PORT omission group than in those that receive PORT in randomized trials [19].
As expected, LR was the most important failure pattern in our study population. Interestingly, recurrence developed only in the refused group, although it should be noted that the follow-up period was relatively short. The patients who were recommended for PORT omission did not receive chemotherapy, but all hormone receptor-positive patients received endocrine therapy.
The recurrence rate in the refused group was higher than that reported in a previous prospective trial [24]. This discrepancy might be associated with differences in the use of optimal systemic management. Many patients enrolled in the present study did not follow the physician's recommendation about either PORT or systemic management. Chemotherapy was used in less than one-third of patients in the refused group, and endocrine therapy was used in less than one-half of those who were hormone receptor positive. Moreover, some patients refuse salvage treatment or adjuvant treatment even after recurrence, despite their potential to be cured [25]. Noncompliance might be an important issue in these patients.
In the recent report of Badakhashi et al. [9], the compliance with PORT was closely related with usage of endocrine therapy, and the 5-year LRFS in the noncompliance group was 75.4%. Although a direct comparison of this recurrence rate with that in our study is not possible because other important factors, such as stage or grade, were not matched, the absolute value was very similar to our 5-year LRFS of 75.5%.
To improve clinical outcomes, therefore, overcoming patient noncompliance might be one of the most important issues. More detailed, accurate explanations of the effect and/or necessity of PORT, consequence of clinical outcomes, and recommended alternatives should be provided to allow the patient to contribute to decision making regarding the optimal management strategy.
This study has several limitations. First, selection bias is inevitable because of the retrospective study design and involvement of a single institution. Second, clinical outcomes of some patients could not be assessed because of early follow-up loss, although the records of all patients who did not receive PORT were analyzed. Third, the assessment of survival outcomes, especially in patients with LR, was not conducted owing to the tendency of early follow-up loss of these patients.
To our knowledge, this is the first report describing the clinical outcomes of PORT omission after BCS in a Korean population. The rate of PORT omission was very low among women who underwent surgery in the present study compared with that of other studies worldwide. Our data indicate that PORT might be safely omitted in carefully selected patients aged 70 years or older. However, the rate of LR was dramatically elevated in the refused group, and solutions to enhance compliance in these patients should be discussed.
Figures and Tables
 | Figure 1CONSORT diagram.BCS=breast-conserving surgery; PORT=postoperative radiotherapy; SMC=Samsung Medical Center; r/o=rule out.
|
 | Figure 2Annual proportion of postoperative radiotherapy (PORT) omission after breast-conserving surgery (BCS). The rate increased slightly to approximately 1.5% after 2006, when physicians started to recommend PORT omission. |
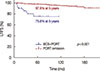 | Figure 3Kaplan-Meyer curves of local recurrence-free survival (LRFS) according to the postoperative radiotherapy (PORT) or omission. When compared with patients who received PORT after breast-conserving surgery (BCS) in 1994 to 2007 at Samsung Medical Center, LRFS was clearly inferior in PORT omission group. |
 | Figure 4Kaplan-Meyer curves of local recurrence-free survival (LRFS) according to prognostic factors. Significantly higher LRFS was detected for patients with histologic grade (HG) 1 or 2 (A), no axillary lymph node (LN) metastasis (B), endocrine therapy (Tx) (C), and the recommended group (D). |
Table 1
Characteristics of patients according to type of postoperative radiotherapy omission

ALN=axillary lymph node; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth receptor 2.
*Median (range); †Analysis was performed in only accessible patients (ER/PR examinations were not possible due to no residual tumor after breast-conserving surgery in referred patients or no additional fluorescence in situ hybridization in HER2); ‡Luminal A like (positive for ER or PR, and negative for HER2); luminal/HER2+ (positive for ER or PR, and positive for HER2); HER2+/ER-PR- (negative for ER/PR, and positive for HER2); triple-negative (negative for all ER, PR, and HER2).
Table 2
Possible prognostic factors predicting local recurrence-free survival

References
1. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011; 61:409–418.

2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

3. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123.

4. Korean Breast Cncer Society. Breast Cancer White Paper. Seoul: Korean Breast Cancer Society;2013.
5. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011; 378:1707–1716.

6. Cinical practice guidelines in oncology: breast cancer. National Comprehensive Cancer Network;2014. Accessed October 1st, 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
7. National Institute for Health and Clinical Excellence. National Collaborating Centre for Cancer. Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. London: National Institute for Health and Clinical Excellence;2009.
8. Nattinger AB, Hoffmann RG, Kneusel RT, Schapira MM. Relation between appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. 2000; 356:1148–1153.

9. Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med. 2013; 2:712–717.

10. White J. Do we need to irradiate all small invasive breast cancers and DCIS? Am Soc Clin Oncol Educ Book. 2013; 40–44.

11. Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Viale G, Renne G, et al. Minimal and small size invasive breast cancer with no axillary lymph node involvement: the need for tailored adjuvant therapies. Ann Oncol. 2004; 15:1633–1639.

12. Kim H, Noh JM, Choi DH, Lee J, Nam SJ, Lee JE, et al. Excision alone for small size ductal carcinoma in situ of the breast. Breast. 2014; 23:586–590.

13. Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004; 351:971–977.

14. Lim YJ, Kim K, Chie EK, Han W, Noh DY, Ha SW. Treatment outcome of ductal carcinoma in situ patients treated with postoperative radiation therapy. Radiat Oncol J. 2014; 32:1–6.

15. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008; 14:1368–1376.

16. Showalter SL, Grover S, Sharma S, Lin L, Czerniecki BJ. Factors influencing surgical and adjuvant therapy in stage I breast cancer: a SEER 18 database analysis. Ann Surg Oncol. 2013; 20:1287–1294.

17. Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002; 20:4141–4149.

18. Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004; 351:963–970.

19. Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013; 31:2382–2387.

20. Tuttle TM, Jarosek S, Habermann EB, Yee D, Yuan J, Virnig BA. Omission of radiation therapy after breast-conserving surgery in the United States: a population-based analysis of clinicopathologic factors. Cancer. 2012; 118:2004–2013.

21. Bane AL, Whelan TJ, Pond GR, Parpia S, Gohla G, Fyles AW, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol. 2014; 25:992–998.

22. Zhou W, Jirström K, Amini RM, Fjällskog ML, Sollie T, Lindman H, et al. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer. 2013; 13:512.

23. Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012; 14:R83.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download