Abstract
Primary neuroendocrine carcinoma of the breast (NECB) is a very rare type of invasive breast carcinoma. Most NECBs appear on breast imaging as solid masses of varied shapes and margins, and have worse clinical outcomes than does invasive ductal carcinoma, not otherwise specified. However, there have been no reports to date regarding NECB with features of inflammatory breast carcinoma. Here, we describe the clinical, radiol-ogic, and pathologic findings of the first reported case of primary NECB presenting as inflammatory breast carcinoma. The patient complained of diffuse right breast enlargement and erythema. Mammography identified severe breast edema and axillary lymphadenopathy. Ultrasound detected an irregular, angular, hypoechoic mass with dermal lymphatic dilatation. On magnetic resonance imaging, the mass had rim enhancement and the entire right breast showed heterogeneous enhancement with malignant kinetic features. Pathology identified the mass as a primary NECB with positive for synaptophysin, CD56, estrogen and progesterone receptors.
Primary neuroendocrine carcinoma of the breast (NECB) is an extremely rare tumor, accounting for 0.27% to 0.5% of histopathologically proven breast cancers [12]. It exhibits morphological features similar to those of neuroendocrine tumors of both the gastrointestinal tract and lung [3]. The standard diagnostic method for NECB is immunohistochemical staining for neuroendocrine markers, including synaptophysin, chromogranin, and neuron-specific enolase [23]. The 2003 World Health Organization (WHO) histologic classification of tumors of the breast and female genital organs defined NECB as breast carcinoma with more than 50% of the cell population expressing these neuroendocrine markers. Furthermore, the diagnosis of primary neuroendocrine carcinoma of the breast can be established when either an in situ component is found or extramammary sites are excluded [45]. In 2012, the WHO histologic classification was upgraded and these tumors were divided into three subtypes: neuroendocrine carcinoma, well-differentiated; neuroendocrine carcinoma, poorly-differentiated/small cell carcinoma; and invasive breast carcinoma with neuroendocrine differentiation [6].
There have been some publications describing the pathologic findings in NECB, but few regarding the radiologic findings. Furthermore, there is debate regarding clinical outcomes for NECB. Some authors reported clinical outcome similar to that of invasive ductal carcinoma (IDC), while other large-scale population studies showed higher local recurrence rates and worse overall survival, compared with IDC [278].
To our knowledge, there has been no report of NECB with clinical features of inflammatory breast carcinoma at the time of diagnosis. In this article, we report the radiologic and pathologic findings of the first reported case of primary NECB presenting as inflammatory breast carcinoma, with a brief review of the relevant literature.
A 48-year-old woman presented to our clinic with diffuse enlargement and erythema of the right breast of 2 weeks duration. She had no risk factors for breast cancer and was taking only oral medication for diabetes mellitus. On physical examination, the right breast was diffusely enlarged and the skin had a peau d'orange appearance. Mediolateral oblique and craniocaudal mammograms showed diffuse skin thickening and edema of the right breast, dense dystrophic calcification in the right subareolar area, and enlarged lymph nodes in both axillae (Figure 1). Breast ultrasound (US) examination was performed with a 6-15-MHz linear transducer (LOGIQ 9 unit; GE Medical Systems, Milwaukee, USA). On US, the normal architecture of breast parenchyma was lost in the right breast. The skin thickness reached up to 6 mm thick and the dermal lymphatics were dilated (Figure 2A). A 24×14 mm, irregular-shaped, angular-margined, hypoechoic mass was observed in the right 9 o'clock position (Figure 2B). Multiple level I and II lymph nodes were enlarged in the right axilla, with level I enlargement in the left axilla (Figure 2C). The lymph nodes had cortical thickening or loss of the fatty hilum. The largest level I node in the right axilla measured 15 mm. These findings were compatible with inflammatory breast cancer with axillary lymph node metastasis.
To assess the tumor extent, magnetic resonance imaging (MRI) was performed using a 3T MRI unit (MAGNETOM Skyra 3.0 T; Siemens Healthcare, Erlangen, Germany). The precontrast T2-weighted scan demonstrated an indistinct, irregular high signal intensity mass in the right 9 o'clock position with skin and chest wall edema (Figure 3A). On the postcontrast T1-weighted image, the mass in the right 9 o'clock position was irregular, and had internal rim enhancement with skin invasion (Figure 3B). The parenchyma of the entire right breast showed heterogeneous enhancement with early rapid and delayed washout enhancement, suggestive of extensive involvement of the cancer.
US-guided core needle biopsy was performed for the right 9 o'clock mass. The pathologic examination of the core biopsy specimen revealed irregular nests or interconnected trabecular formation of tumor cells with fine granular chromatin and indistinct nucleoli on hematoxylin and eosin stain (Figure 4A). There was no other component suggestive of a different subtype of invasive breast carcinoma, such as the mucinous or not otherwise specified type. Immunohistochemical staining was positive for synaptophysin and CD56 (neural cell adhesion molecule) (Figure 4B, C). In addition, the tumor was positive for estrogen receptor and progesterone receptor, but negative for human epidermal growth factor receptor (Figure 4D). The Ki-67 labeling index was 10% to 20%. These pathologic findings were compatible with a diagnosis of primary NECB, poorly-differentiated carcinoma.
The patient underwent chemotherapy, with six cycles of cisplatin and etoposide, six cycles of docetaxel, and three cycles of eribulin. However, on follow-up imaging, the primary cancer in her right breast and metastatic ipsilateral axillary lymph nodes were enlarged, and new metastatic lesions had developed in the left breast. She received endocrine therapy with goserelin acetate and tamoxifen, plus palliative radiation therapy. On follow-up MRI images, multicentric enhancement of the right breast had partially regressed, suggesting a partial response to radiation therapy and/or endocrine therapy. However, multifocal metastatic lesions in the left breast and metastatic axillary lymph nodes had progressed.
There are a few reports of the radiologic findings in primary NECB [145]. Based on these reports, all primary NECBs present as solid masses. On mammography, the tumors were hyperdense, round or irregularly shaped masses with variable margins that were spiculated, indistinct, circumscribed, or obscured [145]. On US, the tumors were oval or irregularly shaped, heterogeneous or homogeneous hypoechoic masses with variable margins, as in mammography. Most masses had normal US transmission [145]. A few reports of MRI findings in primary NECB demonstrated that the tumors were irregular masses with rim enhancement and malignant kinetic characteristics, and early rapid and delayed washout enhancement [4,5].
In our case, the radiologic findings were unique in that the tumor was accompanied by features of inflammatory breast carcinoma. Inflammatory carcinoma is a form of breast carcinoma with a distinct clinical presentation including diffuse erythema, edema, peau d'orange appearance, tenderness, induration, warmth, enlargement, and a palpable ill-defined mass in some cases [6]. It has prominent dermal lymphatic infiltration from an underlying invasive carcinoma, and is a form of advanced breast carcinoma classified as T4d in the TNM classification [9]. In the majority of cases, the underlying carcinoma is diagnosed as IDC, not otherwise specified, but any histologic type of carcinoma can be present. We believe our patient is the first reported case of primary NECB. The case showed typical clinical and radiologic features of inflammatory breast carcinoma. She complained of enlargement, erythema, and peau d'orange appearance of the entire right breast, and radiological examination revealed diffuse skin thickening, dilated dermal lymphatics, and extensive cancer involvement of the right breast on mammography, US, and MRI. On mammography, we could not identify a discrete mass because of severe breast edema. US and MRI demonstrated breast parenchymal findings in detail. An angularmargined, irregular-shaped, hypoechoic mass was found in the 9 o'clock position of her right breast on US. MRI demonstrated that the mass had rim enhancement with early rapid and delayed washout, a malignant kinetic feature. In addition, MRI visualized extensive right breast involvement with heterogeneous enhancement. Axillary lymph node metastases were also found on radiological examination.
With regard to clinical outcome, several recent studies showed higher local recurrence and worse overall survival for NECB than for IDC [278]. In addition, patients with NECB tend to present at an older age (mean age, 65 years) and with a higher T stage (stage T2 in 50.5% of patients) than those with IDC (54 years, stage T1 in 59.2%) at the time of diagnosis, according to the most recent large-scale investigation [2]. Although there have been reports that the incidence of advanced NECB cases presenting with skin or chest wall invasion (stage T4) is 0% to 8%, there has been no reported case of NECB with clinical features of inflammatory carcinoma [27].
The treatment of NECB is not standardized and most patients receive conventional breast cancer management. Therefore, surgery may be considered as first-line therapy when possible [4]. A few case studies have demonstrated the effectiveness of endocrine therapy for hormone receptor-positive NECB or of platinum-based chemotherapy for small cell NECB [1011]. A recent large-scale study of 74 patients with NECB indicated that endocrine and radiation therapy tended to improve overall survival, while chemotherapy had the opposite effect. However, none of these differences reached statistical significance [7]. In our case, chemotherapy with various regimens was attempted first, because of the advanced stage at diagnosis. However, the response was poor. The primary cancer progressed and new metastatic lesions developed in the contralateral breast. As second-line treatment, endocrine therapy with palliative radiation therapy was attempted considering the expression of estrogen and progesterone receptors, but metastatic cancer involving the contralateral breast and axilla progressed. According to recent prognostic research about NECB, higher T and M classification, advanced TNM stage, increased expression of Ki-67, and the absence of progesterone expression all are associated with poor prognosis [2]. Presentation as inflammatory breast carcinoma (T4d stage) and node metastases at diagnosis were poor prognostic factors in our case.
In conclusion, primary NECB is extremely rare, and most NECBs have been detected as solid masses. In this article, we describe the clinical, radiological, and pathological findings of the first reported case of primary NECB presenting as inflammatory breast carcinoma. Inflammatory breast carcinoma presents with dermal lymphatic infiltration by cancer cells. Although the majority of the underlying malignancy in inflammatory breast carcinoma is IDC, we should be aware that NECB, which is an extremely rare malignancy, can present with unique symptoms and radiologic features suggestive of an inflammatory breast lesion.
Figures and Tables
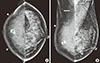 | Figure 1Mammographic findings. Craniocaudal (CC) view (A), mediolateral oblique (MLO) view (B). Both CC and MLO views show severe skin thickening (arrowheads) in the right breast. There is dense dystrophic calcification in the right subareolar area. Enlarged lymph A B nodes (arrows) are also noted in both axilla. |
 | Figure 2Breast ultrasound (US) findings. (A) A breast US image shows diffuse skin thickening and dilatation of dermal lymphatics (arrowheads). (B) There is an angular-margined, irregular-shaped, hypoechoic mass (arrows) in the 9 o'clock position of the right breast. (C) There are enlarged lymph nodes with loss of internal fatty hila in the right axilla (arrows). |
 | Figure 3Breast magnetic resonance imaging findings. (A) Axial precontrast T2-weighted image shows an indistinct irregular high signal intensity mass (arrow) in the right 9 o'clock position and diffuse skin and chest wall edema (arrowheads). (B) Sagittal postcontrast T1-weighted image shows the mass (arrows) in the right 9 o'clock direction with rim enhancement. Multiple enlarged lymph nodes (white arrowheads) are seen in the right axilla. In the right subareolar area, there is a dark round lesion which corresponds to a calcification (black arrowhead). The entire breast parenchyma has heterogeneous enhancement. |
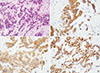 | Figure 4Pathologic findings of the core biopsy specimen. (A) H&E staining shows irregular nests or interconnected trabecular formation of tumor cells with fine granular chromatin and indistinct nucleoli (×400). On immunohistochemistry, tumor cells show diffuse positive staining for synaptophysin (B), CD56 (C), and estrogen receptor markers (D) (×400). |
References
1. Kim JW, Woo OH, Cho KR, Seo BK, Yong HS, Kim A, et al. Primary large cell neuroendocrine carcinoma of the breast: radiologic and pathologic findings. J Korean Med Sci. 2008; 23:1118–1120.

2. Zhang Y, Chen Z, Bao Y, Du Z, Li Q, Zhao Y, et al. Invasive neuroendocrine carcinoma of the breast: a prognostic research of 107 Chinese patients. Neoplasma. 2013; 60:215–222.

3. Ellis IO, Schnitt SJ, Sastre-Garau X. Invasive breast carcinoma. In : Tavassoli FA, Devilee P, editors. International Agency for Research on Cancer. World Health Organization. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press;2003. p. 13–59.
4. Valentim MH, Monteiro V, Marques JC. Primary neuroendocrine breast carcinoma: a case report and literature review. Radiol Bras. 2014; 47:125–127.

5. Günhan-Bilgen I, Zekioglu O, Ustün EE, Memis A, Erhan Y. Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histopathological findings. Eur Radiol. 2003; 13:788–793.

6. Bussolati G, Badve S. Carcinomas with neuroendocrine features. In : Lakhani SR, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press;2012. p. 62–63.
7. Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, et al. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010; 116:4463–4473.
8. Kwon SY, Bae YK, Gu MJ, Choi JE, Kang SH, Lee SJ, et al. Neuroendocrine differentiation correlates with hormone receptor expression and decreased survival in patients with invasive breast carcinoma. Histopathology. 2014; 64:647–659.

9. Breast cancer staging, 7th edition. American Joint Committee on Cancer;Accessed April 24th, 2015. https://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download