Abstract
Adenomyoepithelioma (AME) of the breast is an uncommon tumor characterized by its dual differentiation into luminal cells and myoepithelial cells. In most cases these tumors have a benign clinical course, but distant metastases have been reported. We present the case of a 51-year-old woman diagnosed with malignant AME. The patient underwent a right modified radical mastectomy, and pathological examination confirmed the diagnosis of malignant AME. Ten months after the operation, multiple hepatic, pleural, and abdominal wall metastases were detected. A number of palliative chemotherapeutic agents were tried, including anthracycline and taxanes. However, the disease continued to progress, and superior vena cava syndrome developed as a result of direct tumor invasion. The patient received salvage eribulin monotherapy. After two cycles of this treatment, her clinical symptoms were ameliorated, and a computed tomography scan showed a partial response. Eribulin chemotherapy was thus effective in treating malignant AME in this case.
Adenomyoepithelioma (AME) of the breast is an uncommon tumor characterized by biphasic proliferation of both inner epithelial and outer myoepithelial cells, first described by Hamperl in 1970 [1]. This tumor may display a heterogeneous pattern because of the variable proliferation of epithelial and myoepithelial cells. Although most AMEs are benign, sporadic malignant AMEs with distant metastases have also been reported [234567]. Typically, malignant AME is a large tumor that often originates as a longstanding, stable mass, but then undergoes a period of rapid growth [4].
Eribulin mesylate is a recently approved therapeutic option for patients with metastatic breast cancer [8]. This new agent has a unique mechanism of action, with a tubulin binding site that appears to be different from the taxane and vinca binding sites on the positive end of the microtubule. It has demonstrated efficacy in heavily pretreated patients with metastatic breast cancer, with a largely acceptable toxicity profile [9].
In the case discussed here, a malignant AME arose de novo in the absence of a low-grade precursor lesion. We present the case of a 51-year-old woman with multiple metastases from AME after mastectomy and discuss the effectiveness of salvage eribulin chemotherapy for malignant AME.
A 51-year-old woman presented with a tumor of the right breast. Examination of a core needle biopsy specimen revealed invasive ductal carcinoma. The positron emission tomographic (PET) scan showed an isolated mass in the right breast. The patient underwent right mastectomy with axillary lymph node dissection. Histopathological analysis of the mass showed a biphasic population of both myoepithelial cells and ductal epithelial cells (Figure 1A). Atypia was obvious in both myoepithelial and ductal epithelial cells with a moderate degree of nuclear pleomorphism, prominent nucleoli, a high nuclear-cytoplasmic ratio, and increased mitotic figures (Figure 1B). The ductal epithelial cells were strongly positive for pancytokeratin (pan-CK), cytokeratin 5/6 (CK5/6) (Figure 1C), vimentin, and E-cadherin, but negative for p63 and S100 protein. The myoepithelial cells were strongly positive for p63 (Figure 1D), vimentin, and S100 protein, but negative for CK5/6 and E-cadherin. The tumor cells were strongly positive for p53, and 90% were positive for Ki-67. Lymphovascular invasion was observed (Figure 1E). Tests for both estrogen and progesterone receptors were negative, and human epidermal growth factor receptor 2 amplification was not detected. The histological diagnosis was malignant AME of the breast.
Ten months after the operation, multiple hepatic, pleural, and abdominal wall metastases were detected on follow-up PET/computed tomography (CT). The patient underwent 12 cycles of paclitaxel (175 mg/m2 on day 1, triweekly) plus carboplatin (area under the curve of 5 on day 1, triweekly), eight cycles of vinorelbine (25 mg/m2 on days 1 and 8, triweekly) plus cisplatin (60 mg/m2 on day 1, triweekly), seven cycles of anthracycline (60 mg/m2 on day 1, triweekly) plus cyclophosphamide (600 mg/m2 on day 1, triweekly), and 17 cycles of docetaxel (75 mg/m2 on day 1, triweekly) for 3 years, and the best response to each of these chemotherapeutic regimens was a partial response. Both gemcitabine monotherapy (1,000 mg/m2 on days 1 and 8, triweekly) and capecitabine monotherapy (2,500 mg/m2 on days 1-14, triweekly) were not effective in controlling the disease, which progressed for one cycle.
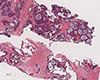
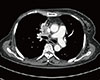
Physical examination showed edema of the face and upper extremities, and the patient complained of mild dyspnea and abdominal pain due to the mass lesion. Chest CT showed invasion of the superior vena cava (SVC) as a result of mediastinal pleural metastasis (Figure 2), and SVC syndrome was diagnosed clinically. A biopsy specimen from the metastatic site was obtained, and the abdominal mass was histologically diagnosed as a metastatic lesion from malignant AME of the breast (Figure 3). To control both the abdominal pain and pleural metastases, treatment with eribulin (1.4 mg/m2 on days 1 and 8, triweekly) was commenced as seventh-line chemotherapy at the patient's request. Her symptoms were relieved after one cycle of eribulin therapy. Two months later, the abdominal mass and pleural metastases had shown a dramatic response (Figure 4), and this response persisted for 6 months.
AME of the breast is a rare disorder characterized by the simultaneous proliferation of ductal epithelium and myoepithelial cells [1]. In cases in which the tumor shows biphasic proliferation of both myoepithelial and epithelial cell components, the term "AME" is used. Although most of these tumors have a benign clinical course, local recurrences, malignant transformations, and distant metastases have been reported. The diagnoses of malignancy were convincingly supported by high mitotic figures, pleomorphism, and invasion in tissue sections [10]. The present case showed atypia, high mitotic rates, and lymphovascular invasion, and was therefore diagnosed as malignant AME.
The interplay between epithelial and myoepithelial cell elements is highlighted by immunohistochemical staining with antibodies specific for these two components. The myoepithelial component is highlighted by p63, smooth muscle myosin heavy chains, and S100 [1112]. Proliferative activity as determined by the proliferative index using Ki-67 immunostaining is present in both compartments of the tumor but may be higher in the myoepithelial cells than in the ductal cells [13]. However, the precise pattern of staining of the myoepithelial cell component is not completely predictable and varies from case to case as well as in different areas within the same tumor [711]. In this case, CK5/6 staining was positive in the epithelial cells and negative in the myoepithelial cells; however, high-molecular-weight cytokeratins (CK14 and CK5/6) can be used as myoepithelial markers [11].
The diagnosis of AME on a needle core biopsy can be challenging because of the tumor's morphologic heterogeneity. When the biopsy material is limited, the sampled tissue may even be mistaken for invasive carcinoma, especially in tumors that have compact glandular structures, as occurred in this case. Therefore, excisional biopsy is recommended to rule out a carcinoma arising within an AME [10]. Either the epithelial, myoepithelial, or both components of an AME may give rise to a carcinoma. A malignant AME in which both cellular components undergo malignant transformation is exceptionally uncommon [235]. In the present case, both cellular components showed malignant features with high Ki-67 expression, and the histopathological features of the metastatic tumor were similar to those of the primary malignant AME in the breast.
The differential diagnosis of AME includes papilloma with myoepithelial hyperplasia, fibroadenoma, phyllodes tumor or tubular adenoma with AME-like areas, invasive ductal carcinoma, ductal adenoma, and nodular adenosis. Some areas of an AME may resemble adenoid cystic carcinoma of the breast, but that entity has infiltrative borders and a characteristic cribriform architecture in most cases. The myoepithelial cells of an adenoid cystic carcinoma tend to be smaller and more hyperchromatic with a basaloid appearance, and have much less cytoplasm than do those of an AME [712]. Immunohistologically, CD117 highlights the epithelial cells of adenoid cystic carcinoma but is completely negative in malignant AME [14].
Most AMEs can be treated by local excision, and complete excision with appropriate margins is recommended to prevent local recurrence [12]. If the lesion recurs, a wider excision would be required. Mastectomy or breast-conserving surgery with radiation and axillary dissection are not necessary for benign AMEs but may be indicated for a carcinoma arising from an AME. However, the treatment for metastatic AME has not been determined, and the prognosis of malignant AME with distant metastases has been very poor, with the time of recurrence varying after initial treatments [3456]. Chemotherapy might play a major role in the treatment of metastatic AME, as has been shown in invasive ductal carcinoma of the breast. In the present case, the patient had received multiple chemotherapeutic regimens, including an anthracycline and taxane, for 3 years.
Eribulin mesylate is a novel, nontaxane inhibitor of microtubule dynamics and has been efficacious in patients with heavily pretreated metastatic breast cancer [89]. Our patient's clinical history suggested that the malignant AME was aggressive but also quite responsive to different lines of chemotherapy, including alkylating agents, anthracyclines, and taxanes. The disease was resistant to gemcitabine and capecitabine, as evidenced by tumor progression during such treatment. Specifically, this patient had a good response to the microtubule inhibitor initially, but prolonged use of the chemotherapy led to resistance [15]. Eribulin has demonstrated marked activity in paclitaxel-resistant ovarian cancer cell lines. In the present case, the patient had symptomatic disease in the form of SVC syndrome and pain, and the disease had progressed during other cycles of chemotherapy. However, eribulin monotherapy relieved her symptoms and resulted in an effective response, with no worsening of her neuropathic symptoms. Considering the many lines of therapy this patient had already received, the results here confirm that eribulin should be considered a feasible treatment in this setting.
Biphasic malignant AME of the breast is a rare neoplasm, and metastasis from both epithelial and myoepithelial cells appears to be very rare. We present the case of a 51-year-old patient with multiple metastases from AME after mastectomy and demonstrate the effectiveness of salvage eribulin chemotherapy for malignant AME in this case. The results suggest that eribulin treatment may be beneficial for patients with metastatic malignant AME.
Figures and Tables
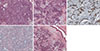 | Figure 1Malignant adenomyoepithelioma of the breast. (A) Biphasic proliferation of both inner eptithelial and outer myoepithelial cells was shown (H&E stain, ×200). (B) Atypia was obvious in both myoepithelial and ductal epithelial cells with moderate degree of nuclear pleomorphism, prominent nucleoli, high nuclear cytoplasmic ratio and increased mitotic figures (H&E stain, ×400). (C) The ductal cells were positive for CK5/6 (immunoperoxidase, ×40). (D) The myoepithelial cells were positive for p63 (immunoperoxidase, ×40). (E) The tumor showed lymphovascular invasion (H&E stain, ×100). |
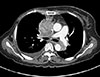 | Figure 2Radiologic findings for chest computed tomography (CT). CT scan showed marked enlarged recurred mass lesion in right mediastinal pleural areas, which invaded to right pulmonary trunk and superior vena cava. |
References
1. Hamperl H. The myothelia (myoepithelial cells): normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970; 53:161–220.
2. Loose JH, Patchefsky AS, Hollander IJ, Lavin LS, Cooper HS, Katz SM. Adenomyoepithelioma of the breast: a spectrum of biologic behavior. Am J Surg Pathol. 1992; 16:868–876.
3. Simpson RH, Cope N, Skálová A, Michal M. Malignant adenomyoepithelioma of the breast with mixed osteogenic, spindle cell, and carcinomatous differentiation. Am J Surg Pathol. 1998; 22:631–636.

4. Michal M, Baumruk L, Burger J, Manhalová M. Adenomyoepithelioma of the breast with undifferentiated carcinoma component. Histopathology. 1994; 24:274–276.

5. Rasbridge SA, Millis RR. Adenomyoepithelioma of the breast with malignant features. Virchows Arch. 1998; 432:123–130.

6. Kihara M, Yokomise H, Irie A, Kobayashi S, Kushida Y, Yamauchi A. Malignant adenomyoepithelioma of the breast with lung metastases: report of a case. Surg Today. 2001; 31:899–903.

7. Hayes MM. Adenomyoepithelioma of the breast: a review stressing its propensity for malignant transformation. J Clin Pathol. 2011; 64:477–484.

8. Muñoz-Couselo E, Pérez-García J, Cortés J. Eribulin mesylate as a microtubule inhibitor for treatment of patients with metastatic breast cancer. Onco Targets Ther. 2011; 4:185–192.
9. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomized study. Lancet. 2011; 377:914–923.

10. Ahmadi N, Negahban S, Aledavood A, Daneshbod K, Daneshbod Y. Malignant adenomyoepithelioma of the breast: a review. Breast J. 2015; 21:291–296.

11. Dewar R, Fadare O, Gilmore H, Gown AM. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011; 135:422–429.

12. Yoon JY, Chitale D. Adenomyoepithelioma of the breast: a brief diagnostic review. Arch Pathol Lab Med. 2013; 137:725–729.

13. Koyama M, Kurotaki H, Yagihashi N, Aizawa S, Sugai M, Kamata Y, et al. Immunohistochemical assessment of proliferative activity in mammary adenomyoepithelioma. Histopathology. 1997; 31:134–139.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download