Abstract
Purpose
The purposes our study was to find out any histologic factors associated with negative conversion of axillary lymph node (ALN) after neoadjuvant chemotherapy (NAC). We also evaluated the association between the decrease in size of primary breast tumor and negative conversion of ALN.
Methods
From January 2012 to November 2014, we included 133 breast cancer patients who underwent NAC and who had ALN metastases which were confirmed on fine-needle aspiration or core needle biopsy at initial diagnosis. All 133 patients underwent initial magnetic resonance imaging (MRI) at the time of diagnosis and preoperative MRI after completion of NAC. We measured the longest dimension of primary breast cancer on MRI.
Results
Of 133 patients, 39 patients (29%) showed negative conversion of ALN and of these 39 patients, 25 patients (64%) showed pathologic complete remission of primary breast. On univariate analysis, mean percent decrease in longest dimension, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 status and histologic grade were significantly associated with the ALN status after NAC (p<0.001, p=0.001, p< 0.001, p=0.001, p=0.002, respectively). On multivariate logistic regression analysis, percent decrease in longest dimension (odds ratio, 1.026; 95% confidence interval [CI], 1.009-1.044) and histologic grade (odds ratio, 3.964; 95% CI, 1.151-13.657) were identified as being independently associated with the ALN status after NAC. The area under the receiver operating characteristic curve was 0.835 with the best cutoff value of 80% decrease in longest dimension. Combination of high histologic grade and more than 80% decrease in longest dimension showed 64% sensitivity and 92% specificity.
Axillary lymph node (ALN) metastasis is one of the most significant prognostic factors for survival in breast cancer patients. Sentinel lymph node dissection (SLND) has become the standard practice for nodal staging in patients with early-stage breast cancer. Several studies revealed that SLND accurately evaluated the status of ALN and was associated with decreased morbidity than axillary lymph node dissection (ALND) in clinically node-negative patients [123].
Neoadjuvant chemotherapy (NAC) has become the standard treatment in patients with inoperable locally advanced and large operable breast cancers. It is also widely used in patients with early invasive breast cancer. Rates of pathologic complete remission (pCR) vary from 16% to 20% according to the histologic subtypes of tumor and treatment modalities [456]. Achievement of pCR has been correlated with better disease-free survival and overall survival [789].
ALN metastases can be eradicated by NAC and the initial nodal stage will change substantially after NAC [1011]. Residual metastatic ALNs after NAC are important prognostic factor for disease-free survival [1213], and lymph nodes involvement at the time of surgery, not the initial axillary node stage, is significantly associated with distant disease-free survival [14].
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is known to be the most accurate diagnostic tool for the assessment of tumor response after NAC [1516]. On maximum intensity projection (MIP) images of MRI, it is easier and more accurate to measure the longest dimension of primary tumor than mammography or ultrasound (US).
The purpose of our study was to find out any histologic factors associated with negative conversion of ALN after NAC. We also evaluated the association between the decrease of primary breast tumor and negative conversion of ALN.
Our Institutional Review Board approved this retrospective study (MED-MDB-14-476). From January 2012 to November 2014, 191 consecutive patients with breast cancer underwent NAC. Of 191 patients, 139 patients had ALN metastases which were confirmed on fine-needle aspiration or core needle biopsy at initial diagnosis. We excluded three patients who did not undergo follow-up MRI, one patient who underwent vacuum assisted biopsy in the outside hospital before initial MRI, and two patients who had human epidermal growth factor receptor 2 (HER2)-positive cancer but did not receive trastuzumab treatment. Finally 133 patients were included for the analysis. Patient characteristics are summarized in Table 1. ALND was performed in 127 patients regardless of SLN status. ALND was omitted in six patients who had converted to clinically negative ALN, near complete remission of primary breast tumor on imaging after NAC and had confirmed negative SLN on pathology.
All patients were scheduled for four cycles of adriamycin and cyclophosphamide (AC) regimen (AC, 50 mg/m2 and 500 mg/m2, respectively) followed by four cycles of paclitaxel 175 mg/m2 at a 3-week interval. Of 133 patients, 12 patients underwent the operation after four cycles of NAC because of complete remission of primary breast tumor on MRI (n=3), disease progression (n=2) or little response to NAC (n=7). All 30 patients who had HER2-positive cancer received trastuzumab treatment.
Patients underwent MR examinations three times, at the time of initial diagnosis, after four cycles of adriamycin and cyclophosphamide and after four cycles of paclitaxel. We used a 1.5-T MR system (Signa HDxt; General Electric Medical Systems, Milwaukee, USA) with a dedicated breast coil (8-channel HD breast array; General Electric Medical Systems). An unenhanced coronal fast low-angle shot three-dimensional T1-weighted image was acquired. Gadobutrol (Gadovist; Bayer Schering Pharma, Berlin, Germany) was injected into an antecubital vein at a dose of 0.1 mmol/kg of body weight and at a rate of 3 mL/sec, followed by a 20-mL saline flush for all patients. Subsequently, five consecutive contrast-enhanced series were acquired. The imaging parameters were repetition time (msec)/echo time (msec) of 5.1/2.4, flip angle of 10°, field of view of 300×300 mm, image matrix of 300×300 pixels, section thickness of 1.5 mm, and section gap of 0 mm.
Subtraction images were reconstructed by subtracting the precontrast images from the early peak postcontrast image obtained at 60 seconds after contrast injection. MIP reconstructions were applied to the subtraction images. Two radiologists with 11 and 7 years' experiences interpreted MRI before and after NAC in consensus. The lesion size was measured as the longest diameter of the lesion on MIP image. In patients with multiple cancers, we recorded the sum of the longest diameter of each lesion.
All patients underwent core needle biopsy before surgery and surgical resection for breast cancer with sentinel lymph node biopsy (SLNB) and/or axillary lymph node dissection after NAC. The localization of primary tumor was performed by charcoal injection after four cycles of NAC. The routinely formalin-fixed, paraffin-embedded tissue blocks of tumors and ALNs were sectioned to 4 µm thickness and stained with hematoxylin and eosin.
The specimens of core needle biopsy were evaluated according to the following histopathologic features: histological type of carcinoma, Black nuclear grade (nuclear grade 1, poorly differentiated; grade 2, moderately differentiated; and grade 3, well differentiated), and modified Bloom-Richardson histological grade (histological grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated). For dichotomous-dependent variables, nuclear grade was classified as high (grade 1) versus low (grades 2 and 3) and histologic grade as low (grade 1 and 2) versus high (grade 3). Expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 was evaluated using standard avidin-biotin complex immunohistochemical staining methods. The ER and PR status were assessed using the Allred score, which was expressed as the sum of the proportion score and the intensity score of positively stained tumor cells. Tumors with an Allred score of at least 3 were regarded as positive. The intensity of HER2 staining was scored as 0, 1+, 2+, or 3+. Tumors with a 3+ score were classified as HER2 positive, and tumors with a 0 or 1+ score were classified as negative. In tumors with a 2+ score, gene amplification by using fluorescence in situ hybridization was used to determine HER2 status.
After completion of NAC, the size and extent of residual cancer were measured. The pCR was defined as the complete disappearance of invasive carcinoma in the breast. Residual ductal carcinoma in situ (DCIS) was included in the pCR category. All specimens were reviewed by an experienced pathologist with 16 years of experience.
The mean values of initial tumor size, percent decrease in longest dimension and duration of NAC were compared using two-sample t-test. Univariate analysis was performed by using chi-square and Fisher exact test for the evaluation of relationships between ALN status with clinical and histopathologic factors. Multivariate analysis was performed using logistical regression of the variables that were found to be statistically significant on univariate analyses. Analyses were performed using the SPSS version 19.0 statistical software package (IBM Corp., Armonk, USA), with a value of p<0.05 considered to be significant.
For the evaluation of diagnostic performance of percent decrease in longest dimension, we used receiver operating characteristic (ROC) analysis. Diagnostic accuracy was calculated from the area under the ROC curve (AUC). The best cutoff was determined from the ROC analysis and we used the best cutoff values to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for predicting negative conversion of ALN. We used MedCalc software (version 10.4.8; MedCalc Software, Ostend, Belgium) for ROC analysis.
Of 133 patients, 39 patients (29%) showed negative conversion of ALN. Of these 39 patients, 25 patients (64%) showed pCR of primary breast tumor and five patients (13%) had residual tumor less than 5 mm.
Univariate analysis was performed for the evaluation of relationships between the negative conversion of ALN and clinical and histological factors (Table 2). Mean percent decrease in longest dimension was significantly higher in patients with negative conversion compared to patients with residual metastatic ALN (82% vs. 37%, p<0.001). There were no significant differences in mean initial tumor size, mean duration of NAC treatment and cycles of NAC (p=0.145, p=0.510, and p=0.508, respectively). ER, PR, HER2 status and histologic grade were significantly associated with the ALN status after NAC (p=0.001, p<0.001, p=0.001, p=0.002, respectively). Nuclear grade was not significantly different between two groups (p=0.222).
Multivariate logistic regression analysis was performed with the variables associated with axillary lymph node status through univariate analysis (Table 3). Percent decrease in longest dimension (odds ratio, 1.026; 95% confidence interval [CI], 1.009-1.044) and histologic grade (odds ratio, 3.964; 95% CI, 1.151-13.657) were identified as being independently associated with the ALN status after NAC. ER, PR, and HER2 were not significant independent factor for ALN status after NAC.
A ROC analysis was performed to differentiate negative conversion group from the residual metastasis group by using percent decrease in longest dimension of primary breast tumor (Figure 1). The AUC was 0.835. The best cutoff for differentiating negative conversion group from residual metastatic ALN group was 80% decrease in longest dimension. With this cutoff, percent diameter decrease showed 89.4% sensitivity, 79.5% specificity, 91.2% PPV, and 73.8% NPV.
Diagnostic performance of combination of high histologic grade or more than 80% decrease in longest dimension was assessed for the diagnosis of metastatic ALN after NAC. The sensitivity, specificity, PPV and NPV were 64% (60/94), 92% (36/39), 95% (60/63), and 100% (36/36), respectively.
Lymph node status after NAC have known to be an important prognostic factor for disease-free survival [121314]. In a recent prospective multicenter study [17], detection rate of SLNB was 80.1% and the false-negative rate was 14.2% in patients with lymph node conversion after NAC. The false-negative rate of SLNB was 12.6%, if two or more sentinel lymph nodes were removed and dual agents were used, in patients with breast cancer with clinical N1 stage receiving NAC in ACOSOG Z1071 trial [18].
However, the detection rate of SLNB after NAC is limited by the effects of NAC, including anatomical alterations or disruptions of lymphatic vessels by tumors, inflammation or fibrosis, blockage by necrotic and/or apoptotic cells or induction of nonuniform tumor regression among ALNs [1920]. In the study of Park et al. [21], patients with poor tumor or nodal response to NAC had higher SLN detection failure rates. They suggested that disruption or blockage of lymphatic pathways by residual tumors might affect the detection rate of radioisotope-based SLNB. Thus, accurate prediction of ALN status before surgery is important because it could help surgeons to proceed directly to ALND or to do SLNB without ALND.
In a recent study analyzing the diagnostic performance of US, MRI, and F-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) for metastatic lymph node after NAC, the sensitivity was 70%, 61%, and 63% and the specificity was 58%, 59%, and 85%, respectively [22]. In a recent review article, the sensitivity of US, MRI, and PET/CT was 58%-86%, 59%, and 48-85%, respectively for the detection of pathologic complete remission of ALN [23]. Previous result of our institution revealed that the sensitivity of US, MRI, and PET/CT was 50%, 72%, and 22% and the specificity of US, MRI, and PET/CT was 77%, 54%, and 85%, respectively [24].
Add of axillary ultrasound (AUS) to SLNB could reduce the false-negative rate from 12.6% to 9.8% in ACOSOG Z1071 trial [25]. In this study, patients with suspicious LNs on AUS had a greater number of positive LNs and larger size of metastasis in ALN and the negative predictive value of AUS was 43% (145/334). In our study, by using the combination of high histologic grade or more than 80% decrease in longest dimension, the PPV and NPV were very high, 95% and 100% for the diagnosis of metastatic ALN. Thus, high PPV could be useful in routine practice for surgeons to proceed to ALND without SLNB and high NPV for surgeons to do only SLNB without ALND.
There are several studies reporting the association of ALN status and the pathologic primary tumor response to NAC. In the study of Nagashima et al. [26], response rate of primary breast tumor was correlated with that of lymph node and these were well correlated with disease-free survival. However, tumor size, histological grade and HER2 were not correlated with patient outcome. In the study of Rouzier et al. [14], high histologic grade and more than 50% response to chemotherapy were associated with negative conversion of ALN after NAC. Our results also showed that high histologic grade and better response of primary breast tumor were associated with negative conversion of ALN.
In the study of Kuerer et al. [27], of 30 patients with pCR of primary breast tumor, 19 patients (63%) had negative ALN at dissection. Thirteen patients (33%) of 40 who had near pCR (residual cancer ≤1 cm3) and 15 patients (17%) of 86 who had residual cancer larger than 1 cm3 showed negative ALN at dissection. Our results also revealed that mean percent decrease in longest dimension was significantly higher in patients with negative conversion compared to patients with residual metastatic ALN (82% vs. 37%, p<0.001). With 80% cutoff value, percent diameter decrease showed 89.4% sensitivity, 79.5% specificity, 91.2% PPV, and 73.8% NPV for the diagnosis of metastatic ALN after NAC.
There are several limitations in our study. First, this was a retrospective study from a single center and the total number of patients was relatively small. Larger multicenter study with more patients is needed to validate our results. Second, we did not evaluate the recurrence of ALN after surgery because of relatively short duration of follow-up. The follow-up study of ALN recurrence is needed for better understanding of cancer biology after NAC.
In conclusion, high histologic grade and more than 80% decrease in primary tumor dimension were associated with negative conversion of ALN after NAC.
Figures and Tables
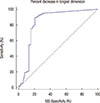 | Figure 1Receiver operating characteristic (ROC) analysis in the differentiation of negative conversion group and residual metastasis group. Area under the ROC curve of percent decrease in longest dimension is 0.835. The best cutoff value is 80% decrease in longest dimension. |
Table 1
Patient and tumor characteristics (n=133)

Table 2
Association of axillary lymph node status after neoadjuvant chemotherapy with clinical and histopathologic prognostic factors

Table 3
Logistic regression analysis for variables associated with negative conversion of axillary lymph node

References
1. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609.

2. Cox CE, Bass SS, Ku NN, Berman C, Shons AR, Yeatman TJ, et al. Sentinel lymphadenectomy: a safe answer to less axillary surgery? Recent Results Cancer Res. 1998; 152:170–179.

3. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663.

4. Ju NR, Jeffe DB, Keune J, Aft R. Patient and tumor characteristics associated with breast cancer recurrence after complete pathological response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013; 137:195–201.

5. Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005; 23:7098–7104.

6. Tanioka M, Shimizu C, Yonemori K, Yoshimura K, Tamura K, Kouno T, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010; 103:297–302.

7. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003; 21:4165–4174.

8. Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003; 21:2600–2608.

9. Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–1466.

10. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.

11. Zhang GC, Liao N, Guo ZB, Qian XK, Ren CY, Yao M, et al. Accuracy and axilla sparing potentials of sentinel lymph node biopsy with methylene blue alone performed before versus after neoadjuvant chemotherapy in breast cancer: a single institution experience. Clin Transl Oncol. 2013; 15:79–84.

12. Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998; 176:502–509.

13. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.

14. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310.

15. Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002; 72:145–152.

16. Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003; 181:1275–1282.

17. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–618.

18. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.

19. Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005; 92:14–23.

20. Brown AS, Hunt KK, Shen J, Huo L, Babiera GV, Ross MI, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph nodepositive breast cancer before treatment. Cancer. 2010; 116:2878–2883.

21. Park S, Park JM, Cho JH, Park HS, Kim SI, Park BW. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol. 2013; 20:2858–2865.

22. Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. 2013; 20:3199–3204.

23. Schipper RJ, Moossdorff M, Beets-Tan RG, Smidt ML, Lobbes MB. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol. 2015; 84:41–47.

24. You S, Kang DK, Jung YS, An YS, Jeon GS, Kim TH. Evaluation of lymph node status after neoadjuvant chemotherapy in breast cancer patients: comparison of diagnostic performance of ultrasound, MRI and (18)F-FDG PET/CT. Br J Radiol. 2015; 88:20150143.
25. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol. 2015; 33:3386–3393.

26. Nagashima T, Sakakibara M, Kadowaki M, Suzuki TH, Yokomizo J, Ohki Y, et al. Response rate to neoadjuvant chemotherapy measured on imaging predicts early recurrence and death in breast cancer patients with lymph node involvements. Acta Radiol. 2011; 52:241–246.

27. Kuerer HM, Newman LA, Buzdar AU, Dhingra K, Hunt KK, Buchholz TA, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998; 4:230–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download