Abstract
Purpose
We investigated the relationships between metastasis-free interval (MFI) and tumor characteristics, and assessed the prognostic value of MFI for survival after metastasis in patients with metastatic breast cancer. Furthermore, we compared MFI among the subtypes.
Methods
We identified 335 patients with postoperative tumor recurrence at distant site(s). All patients underwent curative resection and had a MFI of at least 6 months. MFI was categorized as short (<2 years), intermediate (≥2 years and <5 years), or long (≥5 years). Overall survival after metastasis (OSM) was estimated.
Results
Patients with a shorter MFI were younger, more likely to have initial metastasis to visceral organs, and had a larger tumor with a higher stage and grade as well as a higher rate of nodal involvement at initial diagnosis. Among 136 patients with known disease subtypes, shorter MFI was associated with the triple-negative subtype while longer MFI was associated with the hormone receptor-positive/human epidermal growth factor receptor 2 negative subtype. Mortality after metastasis declined sharply with increasing MFI up to approximately 2 years, and continued gradually declining between 2 and 5 years. An MFI longer than 5 years did not add any survival benefit. MFI was a significant prognostic factor for OSM independent of nodal status, stage, metastatic site, and hormone receptor status of the metastasized cancer.
Metastatic breast cancer (MBC) is generally considered an incurable malignancy that shortens the survival of breast cancer patients. Palliative treatment, which is of limited clinical benefit, remains the most common treatment for MBC in spite of the recent advances in the management of this disease. Therefore, the median survival is only 24 to 30 months after the diagnosis of metastasis [12].
Various prognostic factors have been identified in patients with MBC. A hormone receptor (HR)-negative primary tumor, high histological grade, large tumor size, positive lymph nodes, and old age are all associated with a poor survival outcome, whereas metastases to the bones and soft tissue (as opposed to other sites) are associated with longer survival [123456]. The actual survival rate of individual patients differs extensively owing to the heterogeneity associated with MBC, and its prognosis and clinical course may be dependent on host factors [24]. While recent molecular studies aiming for improved treatment outcome contributed towards the understanding of tumor heterogeneity and the genomic characteristics of MBC [78], this knowledge is not integrated into daily practice and confers only limited survival benefits. In contrast, intrinsic subtypes have been identified by genomic studies, which resulted in therapies tailored to each subtype such as endocrine therapy and human epidermal growth factor receptor 2 (HER2)-targeting therapies in MBC [9].
The metastasis-free interval (MFI) is easy to determine in clinical practice and therefore eliminates the requirement for sophisticated or expensive methods. It was previously reported that MFI is associated with survival after metastasis, and patients with a long MFI have a favorable prognosis even after metastasis [45]. In addition, MFI was reported to be associated with specific tumor characteristics including estrogen receptor (ER) status, suggesting that early recurrence is more prevalent in ER-negative cancer while late recurrence is common for ER-positive cancer [101112]. However, studies investigating the MFI associated with different MBC subtypes are limited in number.
The objectives of this study were to investigate the relationships between MFI and tumor characteristics, and to assess the prognostic value of MFI for survival after metastasis in MBC patients. Furthermore, we compared MFI among the subtypes.
Between April 1989 and May 2008, 2,353 women consecutively underwent surgery for breast cancer. The main patient characteristics, administered treatments, and associated outcomes were documented in the hospital database. Of these 2,353 patients, 438 patients had MBC. Patients with distant metastases at initial assessment (n=72) were excluded. The patients with MFI shorter than 6 months were also excluded because they were considered to have metastatic disease at initial diagnosis (n=31). Among the remaining 335 patients identified to have developed distant metastasis after the primary operation, the disease subtypes for 136 of the patients were determined based on the results of immunohistochemistry analysis.
Formalin-fixed, paraffin-embedded tissue sections obtained from surgical specimens were stained with appropriate antibodies to ER (1:100 clone 6F11; Novocastra, Newcastle upon Tyne, UK), progesterone receptor (PR) (clone 16; Novocastra), HER2 (4B5 monoclonal antibody; Ventana Medical Systems, Tucson, USA), and Ki-67 (MIB-1; Dako, Glostrup, Denmark). The HER2 status was determined through membranous staining using a scoring of 0, 1+, 2+, or 3+ based on the strength of membrane staining [12]. The HER2 status was considered positive if the score was 3+, and as negative, if the score was 0 or 1+. Tumors with a score of 2+ were sent for fluorescence in situ hybridization using the PathVysion HER2 DNA Probe Kit (Abbott-Vysis, Des Plaines, USA). In order to establish the HER2 status in more of the patients, HER2 expression was evaluated using tissues from the primary tumor or the metastatic site. Ki-67 expression was determined in 92 samples as the percentage of positive tumor cells, and stratified as high or low using a cutoff of 14%.
Patients (n=136) were classified into HR-positive/HER2-negative, HR-positive/HER2-positive, HR-negative/HER2-positive, and HR-negative/HER2-negative (i.e., triple-negative [TN]) subtypes based on their tumor expression levels of ER, PR, and HER2.
The staging was performed according to the guidelines of American Joint Committee on Cancer (AJCC), seventh edition. The modified Scarff-Bloom-Richardson grading system was used for tumor grading. Adjuvant systemic therapy and/or radiotherapy were administered according to the standard guidelines based on patient age, primary tumor characteristics, and axillary lymph node status. Endocrine therapy was administered to patients with HR-positive tumors. A 6-month follow-up scheme was advised and if the appointment was missed, the patient was contacted and asked to make a new appointment in order to minimize the risk of failure due to lack of follow-up and to improve the accuracy of survival data. The final update to the clinical database was made in December 2013. Distant metastasis was defined as the recurrence of invasive cancer at distant organs excluding local recurrence (ipsilateral breast or chest wall) and regional recurrence (ipsilateral axillary, infraclavicular, internal mammary, or supraclavicular) and was confirmed using imaging studies and/or tissue biopsies.
The Institutional Review Board (IRB) of Gangnam Severance Hospital approved the study in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki (3-2014-0048). The IRB granted a waiver for written documentation of informed consent from all participants because of the retrospective nature of the study design.
The MFI was defined as the interval between surgery and the date of diagnosis of the first distant relapse, which was longer than 6 months in each case. The MFI was then categorized as short (<2 years), intermediate (≥2 years and <5 years), or long (≥5 years).
Our primary objective was to investigate the relationships between MFI and tumor characteristics. These relationships were compared using a chi-square test. One-way analysis of variation (ANOVA) was performed for comparison of MFIs among tumor subtypes. Our secondary objective was to determine the overall survival after metastasis (OSM), which was calculated from the date of the first distant metastasis to the date of the last follow-up, or until death during the follow-up period. The Kaplan-Meier method was used to estimate OSM, and Cox hazards regression model was used for multivariate survival analysis. In order to investigate the relationship between MFI and the risk of death associated with MBC, restricted cubic spline models, which are smoothly joined piecewise third order polynomials, were used [13]. These polynomials were fitted within intervals delimited by knots, and restrictions were placed on the resulting curve to ensure a smooth appearance at the knot points. A 3-knot analysis was performed. Using Harrell C-statistic, the concordance index (c-index) for time-to-event data was calculated, for which increasing values between 0.5 and 1.0 indicates an increase in the accuracy of the prediction [14]. These analyses were performed using SPSS version 18 (SPSS Inc., Chicago, USA) and R (http://www.r-projet.org) software. Statistical significance was determined by a p-value <0.05 or a 95% confidence interval (CI).
The baseline characteristics of 335 patients with MBC obtained from our analysis are summarized in Table 1. The median ages at diagnosis of the primary tumor and the first metastasis were 44 years (range, 22-85 years) and 47 years (range, 26-85 years), respectively. The majority of patients had tumors larger than 2 cm (72.2%), and axillary metastases (70.7%). The percentage of patients with ER-negative and HER2-positive tumors was 37.3% and 14.0%, respectively. Among the patients with known tumor subtypes, 25.7% were TN. Most patients received adjuvant chemotherapy (85.9%), whereas less than half of the patients received adjuvant endocrine therapy (48.1%) or radiotherapy (41.1%).
The median MFI was 31 months (range, 6-200 months), where 133 patients (40.0%) had a short, and 135 patients (40.0%) had intermediate MFIs. A minority of patients (20.0%) developed metastases 5 years after the initial operation. At the time of the first distant relapse, metastasis was usually confined to a single organ (65.7%), most frequently the bone (23.9%).
The relationships between MFI and clinicopathologic characteristics are summarized in Table 2. Initially, we compared age at metastasis with MFI. More than half of patients aged less than 50 years at first distant relapse had a short MFI, while significantly fewer patients in the long MFI group were aged less than 50 years (p<0.001). Patients with a long MFI were more likely to have bone metastasis, while those with a short MFI more frequently had central nervous system or lung metastasis (data not shown). When metastatic sites were only classified as bone, viscera, or both, patients with a long MFI were still more likely to show bone metastasis, while patients with a short MFI were more likely to have more visceral metastasis.
Investigation into the relationship between MFI and the tumor subtype revealed that approximately 50% of patients with a short MFI had a TN tumor, while more than half of the patients with a long MFI had HR-positive/HER2-negative tumors (p<0.001). The mean MFI was the longest in patients with HR-positive/HER2-negative tumors, and shortest in those with TN tumors (p<0.001) (Figure 1). Furthermore, patients with a short MFI more frequently had larger tumors (p=0.019), nodal involvement (p=0.005), an advanced stage (p<0.001), and a higher grade (p<0.001), although Ki-67 expression in the primary tumor did not vary according to MFI (p=0.233).
The median follow-up duration was 61.0 months (95% CI, 51.7-70.3), during which 247 of the 335 patients died, giving a median OSM of 20.0 months (95% CI, 15.9-24.1). During our analysis of MFI as a continuous variable, we observed that the risk of death after metastasis declined sharply with an increasing MFI up to approximately 2 years, continued gradually declining for MFIs between 2 and 5 years, and showed no significant change for MFIs longer than 5 years (Figure 2). OSM also differed significantly depending on the MFI category (p<0.001) (Figure 3). Patients with a short MFI had a very poor outcome (median OSM, 9.0 months; 95% CI, 5.5-12.5) compared to those with a long MFI (median OSM, 42.0 months; 95% CI, 25.3-58.7).
We concluded from univariate analyses that nodal status, stage, and HR status, were significantly correlated with OSM (Table 3). When adjusted for nodal status, stage, HR status, and metastatic site using the Cox hazards regression model, MFI was significantly associated with the risk of death after metastasis (continuous MFI: HR, 2.39, 95% CI, 1.20-4.76; categorized MFI: HR of intermediate MFI 0.239, 95% CI, 0.174-0.330; HR of long MFI, 0.063, 95% CI, 0.039-0.102) (Table 4). The Harrell c indices for the models with continuous and categorized MFIs were 0.834 and 0.820, respectively.
We concluded from survival analyses that as previously reported [24], the MFI is a significant prognostic marker for survival after metastasis. We report a novel finding that continuous MFI has a prognostic value in multivariate analysis, and therefore can potentially be used to estimate the risk of death after metastasis while previous studies only reported a prognostic value for categorized MFI in multivariate models for OSM [24].
Our investigation into the relationship between MFI and tumor subtype revealed that a shorter MFI was associated with the TN subtype while a longer MFI was associated with the HR-positive/HER2-negative subtype. It was previously reported that the risk of early relapse is greater for women with ER-negative rather than ER-positive breast cancer whereas late relapses are more common in those with ER-positive rather than ER-negative disease [101112]. In this study, we have identified a relationship between the MFI and the tumor subtype, which in turn helps to provide a biological basis for the duration of MFI.
Furthermore, we report that patients with a shorter MFI were more likely to be younger and had visceral organ metastases at the time of diagnosis. These patients often had larger tumors, a higher stage, a higher grade, and nodal involvement at initial diagnosis. We show that shorter MFI is associated with risk factors at the time of diagnosis for both the primary tumor and its metastasis, including the metastatic site.
On the basis of our findings concerning the prognostic value of MFI for OSM, we suggest that upon validation of MFI as a surrogate marker of survival after distant relapse in large trials, it could be incorporated into a potential novel therapeutic strategy for the improvement of survival outcome. Current trials evaluating the effect of new therapeutic interventions aim to reduce events of recurrence or mortality. However, our findings generate a new theoretical model aiming for the prolongation of MFI regardless of decreasing metastatic events.
As previously reported, we show by producing comparable results with a longer median OSM (28.0 months) in HR-positive patients compared to HR-negative patients (12.0 months), that HR status is a prognostic factor for OSM [245]. Previous studies also showed that HR-positive status was associated with a reduced risk of mortality among MBC patients. Other factors affecting OSM were lymph node status (when adjusted with continuous MFI), and location of metastasis. Although our MBC patients had a higher than average tumor burden compared to other breast cancer patients, we found that the nodal status of the primary tumor was a significant prognostic factor for OSM. This may be because of the contribution of many factors towards the overall prognoses of both primary breast cancer and MBC [315].
In addition to lymph node status, we also show that the location of the metastasis is a significant predictive factor for OSM. The median OSM of patients with initial bone-only metastasis was 40 months, which was longer than that of patients with multiple metastases or visceral metastasis at initial distant relapse (20.0 months and 14.0 months, respectively). The favorable OSM in patients with initial bone-only metastasis is also consistent with the findings reported in previous studies [151617].
We acknowledge several limitations inherent to the retrospective design of this study. Palliative treatments were not included in the survival analyses owing to their heterogeneity and information on HER2 status and subtype was missing for more than half of the patients. Consequently, the therapeutic effect of trastuzumab in the palliative or adjuvant setting was not accounted for in this study. In addition, the study was conducted on a small population of patients at a single institute that may not be representative of the general MBC patient population. Nevertheless, we found that MFI is associated with biological traits and may be helpful if used in combination with other prognostic markers.
In conclusion, our findings show that MFI is closely related to tumor characteristics of both primary tumors and their metastases, and that it is a significant prognostic factor for survival after metastasis. In conclusion, we suggest using MFI as an easy to calculate surrogate marker of survival in clinical trials.
Figures and Tables
Figure 1
Comparison of the mean metastasis-free interval (MFI) according to tumor subtype (p<0.001; one-way analysis of variation test).
HR=hormone receptor; HER2=human epidermal growth factor receptor 2; TN=triple-negative.
Figure 2
Relationship between the continuous metastasis-free interval (MFI) and the risk of death after metastasis. The solid curve represents the continuous relationship between MFI and the risk of death after metastasis, based on a univariate spline Cox regression model with three knots. Dotted curves represent 95% confidence intervals.
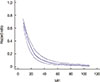
Figure 3
Kaplan-Meier plots for overall survival after metastasis according to metastasis-free interval (MFI) categories (p<0.001; log-rank test).

Table 1
Baseline characteristics (n=335)

AJCC=American Joint Committee on Cancer; DCIS=ductal carcinoma in situ; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; HR=hormone receptor; TN=triple-negative; CNS=central nervous system.
*Median (range); †Data with missing values; ‡HER2 positivity was defined by a 3+ score on immunohistochemistry or amplification on fluorescence in situ hybridization.
Table 2
Relationships between metastasis-free interval and clinicopathologic characteristics

Table 3
Univariate analysis for overall survival after metastasis

Table 4
Multivariate analysis for overall survival after metastasis using the Cox regression hazard method

Notes
References
1. Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999; 56:67–78.

2. Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008; 19:2012–2019.

3. Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003; 97:545–553.

4. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012; 23:103–112.

5. Kwast AB, Voogd AC, Menke-Pluijmers MB, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014; 145:503–511.

6. Planchat E, Durando X, Abrial C, Thivat E, Mouret-Reynier MA, Ferriere JP, et al. Prognostic value of initial tumor parameters after metastatic relapse. Cancer Invest. 2011; 29:635–643.

7. Andre F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014; 15:267–274.

8. Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013; 45:1439–1445.

9. Dignam JJ, Dukic V, Anderson SJ, Mamounas EP, Wickerham DL, Wolmark N. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat. 2009; 116:595–602.

10. Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998; 52:227–237.

11. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996; 14:2738–2746.

12. Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007; 25:5418–5425.

14. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.

15. James JJ, Evans AJ, Pinder SE, Gutteridge E, Cheung KL, Chan S, et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br J Cancer. 2003; 89:660–665.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download