Abstract
Purpose
This study aimed to determine the oncologic efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment concurrent with chemotherapy in a neoadjuvant setting.
Methods
A retrospective analysis was performed on 332 cases of invasive breast cancer in patients who were <40 years old at diagnosis and received GnRH agonists concurrent with neoadjuvant chemotherapy (GnRH agonist group) or neoadjuvant chemotherapy alone (neochemotherapy-alone group) from December 2010 to September 2014. Pathologic complete response rates (pCR) and Ki-67 changes were evaluated between the two groups.
Results
Median age was 32±3.9 and 36±3.0 years in the GnRH agonist group and neochemotherapy-alone group, respectively (p<0.001). After adjustment for tumor size, grade, lymph node metastasis, hormone receptor (HR) status, and chemotherapy regimen, the GnRH agonist group exhibited a higher pCR rate with an odds ratio (OR) of 2.98 (95% confidence interval [CI], 1.37-6.34) and a greater decrease in Ki-67 expression after treatment (p=0.05) than the neochemotherapy-alone group. For HR-negative tumors, the GnRH agonist group showed a higher pCR rate (multivariate OR, 3.50; 95% CI, 1.37-8.95) and a greater decrease in Ki-67 expression (p=0.047). For HR-positive breast cancer, the pCR rate, change in Ki-67 index, and clinical response were higher, and preoperative endocrine prognostic index scores were lower, in the GnRH agonist group, but these did not reach statistical significance.
Early ovarian failure after chemotherapy is an important issue for young breast cancer patients. The desire for fertility preservation and for better survival outcomes may at times conflict, but it is nonetheless a significant issue among these patients.
Trials of gonadotropin-releasing hormone (GnRH) agonists coadministered with adjuvant chemotherapy for the purpose of protecting ovarian function have shown mixed results. However, in negative trials [12] and in trials with a strict primary endpoint, a small number of patients, and patients from different age groups, results have differed [3]. Recently, the Prevention of Early Menopause Study (POEMS/S0230) reported that administration of a GnRH agonist with chemotherapy appeared to protect against ovarian failure, reducing the risk of early menopause and improving prospects for fertility preservation [4]. The results of the POEMS/S0230 trial will lead to important changes in treatment strategies for young breast cancer patients who are considering chemotherapy.
The POEMS/S0230 trial, which included only estrogen receptor (ER) and progesterone receptor (PR) negative tumors, indicated that women treated with GnRH agonists had improved disease-free survival and overall survival rates. Disease risk factors were not stratified in the study, making it difficult to draw conclusions about the oncologic effects of the GnRH agonist. However, an adjustment for breast cancer stage did not alter disease-free survival or overall survival rates. A retrospective study suggested that GnRH agonist treatment concurrent with chemotherapy can be effective, especially against hormone receptor (HR)-positive tumors. However, although a survival benefit was observed, it was not significant [5]. GnRH agonist administration is a promising therapy for fertility preservation, and the oncologic efficacy of GnRH agonist administration with concurrent chemotherapy is an important concern for young premenopausal breast cancer patients.
Therefore, we retrospectively evaluated neoadjuvant responses to determine the oncologic effects of GnRH agonist administration concurrent with chemotherapy in this patient population.
The protocol of the study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2015-0579). The study was designed by the authors. Data were obtained from the Asan Medical Center Breast Cancer Center (AMCBCC) database. The AMCBCC database is a prospectively maintained, web-based system that includes information on all patients who have undergone operations for breast cancer at the Asan Medical Center in Korea since 1989. Patient consent is not necessary for a retrospective study. Inclusion criteria in this study were as follows: (1) newly diagnosed breast cancer from December 2010 to September 2014; (2) patient age under 40 years; (3) a diagnosis of invasive nonmetastatic breast cancer; and (4) having received neoadjuvant chemotherapy. Two treatment groups were analyzed for the study, one received goserelin concurrent with neoadjuvant chemotherapy (goserelin group) for fertility preservation and the other received neoadjuvant chemotherapy alone (neochemotherapy-alone group). For evaluation of neoadjuvant responses, a surgical oncologist reviewed all medical records.
All patients had received neoadjuvant chemotherapy according to the tumor factor. Neoadjuvant chemotherapy was recommended for patients who had positive lymph node involvement, and/or who were expected to benefit from surgery. Positive staining for the ER or PR was defined as a score of more than 3+ and HER2/neu positivity was defined as a score of 3+ by immunohistochemical staining or HER2/neu gene amplification by fluorescence in situ hybridization. The HR-positive group comprised ER-positive and/or PR-positive patients. Patients with HER2-positive breast cancer who received neoadjuvant trastuzumab treatment were excluded from this study. GnRH agonist was given via subcutaneous injection 1 week before the initiation of chemotherapy and administered every 28 days until completion of chemotherapy. The type of GnRH agonist was goserelin (AstraZeneca, London, UK). Surgery was carried out on the basis of the results of ultrasound and/or magnetic resonance imaging which were checked before and after neoadjuvant chemotherapy. For patients who were considered lymph node negative on radiologic examination after neoadjuvant chemotherapy, sentinel lymph node biopsy (SLNB) was performed during surgery, and in those cases of negative SLNB, axillary lymph node dissection was omitted.
The primary endpoint of this study was pathologic complete response, defined as no invasive or in situ tumors of the breast or axilla. The secondary endpoint was the change in the Ki-67 proliferation index before and after neoadjuvant treatment. The changes in this index were determined by comparing the values between core needle biopsy and surgical specimen. For HR-positive breast cancer, clinical responses, including complete response and partial response, were evaluated by ultrasonography according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Preoperative endocrine prognostic index (PEPI) scores [6], scored using pathologic stage, ER status and Ki-67 expression were also evaluated.
The primary analysis was based on logistic regression, stratified according to tumor size, lymph node metastasis, HR status, and chemotherapy regimen. In the subgroup analysis according to HR status, HR status was removed from stratification. Chi-square tests were used to compare baseline clinicopathologic characteristics, changes in the Ki-67 proliferation index (in which a 20% absolute decrease was used as a cutoff value), and the clinical response between the two treatment groups. Differences in mean age and PEPI score were determined by independent t-tests. For evaluation of Ki-67 expression, paired t-tests were used. All data were analyzed using SPSS version 21.0 (IBM Corp., Armonk, USA). All p-values were two-tailed and a p-value of <0.05 was considered statistically significant.
The mean age was 36±3.0 years in the neochemotherapy-alone group and 32±3.9 years in the goserelin group (p<0.001). Clinical T stage, node metastasis, histologic grade, HR status, HER2 status, and chemotherapy were equally distributed between the two groups. The majority of patients received adriamycin and cyclophosphamide (AC) chemotherapy for lymph node-negative disease or AC followed by paclitaxel (T) chemotherapy for lymph node-positive disease (Table 1). Lymph node metastasis was determined using fine-needle aspiration or core needle biopsy before chemotherapy.
The pathologic complete response (pCR) rate was 18.1% in the goserelin group and 10.2% in the neochemotherapy-alone group (p=0.032). Logistic regression analysis after adjusting for tumor size, grade, lymph node metastasis, HR status, and chemotherapy regimen revealed that the odds ratio (OR) of the pCR rate was 2.95 (95% confidence interval [CI], 1.37-6.34). For HR-negative tumors, the pCR rate was 31.5% in the goserelin group and 18.8% in the neochemotherapy-alone group (p=0.067). After adjusting for tumor size, lymph node metastasis, and chemotherapy regimen, the OR was 3.50 (95% CI, 1.37-8.95). However, for HR-positive tumors, the pCR rate was 6.5% in the goserelin group and 4.6% in the neochemotherapy-alone group (p=0.407) and was not significantly different (OR, 1.60; 95% CI, 0.33-7.72) (Figure 1).
The decrease in the Ki-67 proliferation index after chemotherapy was lower in the goserelin group than in the neochemotherapy group (18.6 vs. 25.7, p=0.050). For HR-negative tumors, the decreased Ki-67 proliferation index in the goserelin group was significant (p=0.047), although this was not significant in cases of HR-positive breast cancer (p=0.418).
Using a 20% decrease in the Ki-67 proliferation index as a cutoff value, 48.3% of patients in the goserelin group experienced a decrease of over 20% after neoadjuvant chemotherapy compared with 31% of patients in the neochemotherapy-alone group (p=0.002). The ≥20% decrease in Ki-67 proliferation index in the goserelin group was significant for HR-negative tumors (p=0.004) but was not significant for HR-positive tumors (p=0.078).
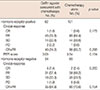
The clinical response based on RECIST criteria version 1.1 was evaluated by ultrasonography. For HR-positive and HR-negative tumors, clinical responses were not significantly different between groups, and for HR-positive tumors, PEPI scores were also not different (Table 2).
The present study showed that GnRH agonist treatment concurrent with chemotherapy resulted in a higher pCR rate and greater decrease in Ki-67 proliferation compared with neochemotherapy alone. The increased pCR rate and reduced Ki-67 index in the GnRH agonist group were significant for HR-negative tumors. For HR-positive tumors, the GnRH agonist group showed a higher pCR, lower Ki-67 index, higher response rate, and lower PEPI score compared to the neochemotherapy alone group, but these differences were not significant. This suggests that GnRH agonist treatment concurrent with chemotherapy results in an improved oncologic response especially in HR-negative tumors. Although significant differences were not obtained with HR-positive tumors, various measures of efficacy showed more favorable results in the GnRH agonist group.
The recent POEMS trial showed that GnRH agonist administration concurrent with chemotherapy resulted in a protective effect on pregnancy and against ovarian failure and ovarian dysfunction [4]. GnRH agonist administration for fertility preservation will likely become a standard treatment, one that is convenient to receive in contrast to embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. Oncologic concerns suggest that it is important to provide GnRH agonist treatment concurrent with chemotherapy for fertility preservation because concurrent use of endocrine therapy and chemotherapy is not recommended by the results of the SWOG-INT-0100 trial [7], which showed that sequential use of tamoxifen compared with concurrent use resulted in increased disease-free survival. However, to date no trials have compared the use of concurrent versus sequential GnRH agonist administration with chemotherapy.
In our study, the GnRH agonist group showed a higher rate of pCR and a markedly decreased proliferation index compared with that of the neochemotherapy-alone group. The POEMS/S0230 trial showed survival benefits on an exploratory survival analysis after adjustment for breast cancer stage in HR-negative tumors [4]. Results from preclinical studies suggest that GnRH-receptor expression in triple-negative tumors might be associated with growth inhibition, reduced metastasis, and apoptotic cell death [89]. However, the mechanism underlying the oncologic efficacy of GnRH agonists or ovarian suppression concurrent with chemotherapy remains unclear. The present study extends the results of the POEMS/S0230 trial and suggests that GnRH agonist treatment concurrent with chemotherapy may be effective in HR-negative tumors.
In HR-positive tumors, the pCR rate and proliferation index were decreased but not to a significant degree. For HR-positive tumors, the pCR was low and found not to be related to survival in a previous neoadjuvant study [10]. Therefore, the neoadjuvant trial could not determine the oncologic efficacy in HR-positive tumors because of the low pCR rate and the absence of any relationship with survival. Understanding how the tumor response is related to relapse risk would help clinicians make decisions about additional treatment options for patients receiving neoadjuvant treatment for ER-positive breast cancer. The present study showed an improved clinical response and low PEPI score in the GnRH agonist group, although this was not significant. PEPI scores are used for the prediction of recurrence on the basis of the integration of pathologic staging parameters and Ki-67 proliferation after neoadjuvant endocrine therapy. A low PEPI score is associated with a low rate of relapse. The results of the present study could not determine the oncologic efficacy in HR-positive tumors, but GnRH agonist treatment with concurrent chemotherapy was not harmful in HR-positive tumors.
However, in HR-positive tumors, chemotherapy-induced amenorrhea confers an improvement in disease-free survival. Therefore, concurrent GnRH agonist treatment and chemotherapy may protect ovarian function, with early restoration of ovarian function, and could be related to poor survival. A joint analysis of the Tamoxifen and Exemestane Trial (TEXT) and Suppression of Ovarian Function Trial (SOFT) showed an excellent survival benefit from adjuvant treatment with exemestane plus ovarian suppression in premenopausal HRpositive tumors [11]. One difference between the TEXT and SOFT was in the timing of ovarian suppression. In the TEXT, ovarian suppression began when patients received chemotherapy, but in the SOFT, ovarian suppression began after the end of chemotherapy when premenopausal serum estradiol was restored. Even though the TEXT included more cases of lymph node-positive tumors, the survival rate in the TEXT was higher than it was in the SOFT. A cross-trial comparison of survival rates between TEXT and SOFT is confounded by the fact that the SOFT chemotherapy cohort began treatment approximately 8 months after surgery and so were further along in their disease time course and therefore not directly comparable to TEXT patients. This suggests, however, that ovarian suppression with chemotherapy and then long-term suppression of ovarian function after chemotherapy might have greater oncologic efficacy in HR-positive breast cancer.
There are several limitations to the present study. First, a small sample size was used. Second, the results of the neoadjuvant trial are not always correlated with the result of same adjuvant trail. A retrospective study reported the oncologic safety of ovarian suppression concurrent with chemotherapy [5]. To determine the oncologic efficacy, we evaluated the neoadjuvant response between two treatment modalities, not only in the total study population but also in a subgroup analysis conducted according to HR status. To overcome the uncertainty of HR-positive tumors, the pCR and proliferation index as well as the clinical response and PEPI scores were evaluated. Third, clinical response was evaluated via ultrasonography RECIST guidelines (version 1.1) do not recommend ultrasonography or response evaluation [12]. Despite these limitations, this study is valuable because many young women with breast cancer struggle with the competing interests of optimizing personal survival and the desire to maintain ovarian function [13]. GnRH agonists are effective for ovarian function preservation and might have oncologic efficacy against breast cancer. If patients can survive cancer as well as preserve their desired quality of life after cancer treatment, they may overcome the fear of breast cancer and cancer treatment.
The findings of this study have clinical implications for women who plan to undergo chemotherapy. Concurrent administration of GnRH agonists during neoadjuvant chemotherapy improved pCR rates and suppressed Ki-67 expression especially in HR-negative tumors. Prospective clinical trials to confirm oncologic efficacy are warranted.
Figures and Tables
 | Figure 1Multivariate odds ratio of pathologic complete response after neoadjuvant chemotherapy between two treatment groups.GnRH=gonadotropin-releasing hormone; CI=confidence interval.
|
Table 1
Baseline characteristics of the patients

Table 2
Clinical response and PEPI score between treatment groups
ACKNOWLEDGMENTS
We all thank all the participants in the study, study personnel for their devoted work during data collection. We also would like to acknowledge Dr. Choi for general support.
References
1. Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011; 29:2334–2341.

2. Munster PN, Moore AP, Ismail-Khan R, Cox CE, Lacevic M, Gross-King M, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo) adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012; 30:533–538.

3. Blumenfeld Z. ZORO study: discrepancy between the conclusion and the results. J Clin Oncol. 2011; 29:3340.

4. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015; 372:923–932.

5. Kim J, Kim M, Lee JH, Lee H, Lee SK, Bae SY, et al. Ovarian function preservation with GnRH agonist in young breast cancer patients: does it impede the effect of adjuvant chemotherapy? Breast. 2014; 23:670–675.

6. Ellis MJ, Tao Y, Luo J, A'Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008; 100:1380–1388.

7. Albain KS, Barlow WE, Ravdin PM, Farrar WB, Burton GV, Ketchel SJ, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:2055–2063.

8. Buchholz S, Seitz S, Schally AV, Engel JB, Rick FG, Szalontay L, et al. Triple-negative breast cancers express receptors for luteinizing hormonereleasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibition. Int J Oncol. 2009; 35:789–796.

9. Schubert A, Hawighorst T, Emons G, Grundker C. Agonists and antagonists of GnRH-I and -II reduce metastasis formation by triple-negative human breast cancer cells in vivo. Breast Cancer Res Treat. 2011; 130:783–790.

10. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.

11. Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014; 371:107–118.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download