Abstract
Purpose
This study investigated the clinicopathological features of operable breast cancer lesions located in different hemispheres of the breast and determined related survival outcomes.
Methods
Data from 5,330 patients with invasive ductal carcinoma were retrospectively analyzed based on tumor location.
Results
The median follow-up time was 68 months (range, 18-176 months). Patients with breast cancer located in the outer hemisphere of the breast had lesions with more advanced nodal stages and more frequently received adjuvant chemotherapy than patients with breast cancer in the inner hemisphere. The 5-year disease-free survival (DFS) rates of patients with tumors located in outer versus inner hemispheres were 81.5% and 77.0%, respectively (p=0.004); the overall survival (OS) rates were 90.7% and 88.8%, respectively (p<0.001). The association between tumor location and the 5-year DFS rate was most apparent in node-positive patients (73.1% vs. 65.8% for outer vs. inner hemisphere lesions, p<0.001) and in patients with primary tumors greater than 2 cm in diameter (78.2% vs. 72.3%, p=0.002). Multivariate analysis showed that tumor location was an independent predictor of DFS (hazard ratio [HR], 1.23; p=0.002) and OS (HR, 1.28; p=0.006). There were no significant differences in 5-year DFS or OS rates between patients with outer versus inner hemisphere tumors when internal mammary node irradiation was performed.
The identification of prognostic and predictive factors for specific treatments continues to be a major area of research. The prognostic role of tumor location in breast cancer has not yet been clarified. There is insufficient evidence to declare that breast tumors located in different hemispheres of the breast have significantly different biological characteristics. However, several recent studies have reported a higher risk of relapse and death among women with primary tumors in the medial breast than that among women with cancer in the lateral breast [1]. It is reasonable to assume that this increased risk may be due to metastases originating from involved but untreated internal mammary nodes (IMNs). Although studies have shown that treatment of IMNs is associated with a small but consistent long-term benefit, current clinical practice does not routinely include surgical removal of IMNs or radiation of the region [2,3,4]. This issue is relevant to radiologic and medical decisions about adjuvant treatment.
This study is a retrospective review of patients with operable breast cancer. The purpose of this study was to evaluate the effect of breast cancer location on survival outcomes and to identify groups of patients who would benefit most from treatment of IMNs.
Data from 5,330 eligible patients with breast cancer who were treated at Sun Yat-Sen University Cancer Center between January 1997 and December 2008 were retrospectively analyzed. The recruitment criteria have been described previously [5]. In brief, eligibility criteria included invasive ductal carcinoma, nonmetastatic breast cancer at diagnosis, availability of complete medical records, and at least 12 months of follow-up. Patients with tumors located in the central portion of the breast and the nipple were excluded (n=479). All patients were staged according to the American Joint Committee on Cancer Tumor-Node-Metastasis Staging System for Breast Cancer (AJCC 2010, 7th edition). The Institutional Review Board and academic committee of Sun Yat-Sen University Cancer Center reviewed and approved this study (IRB number: B2014-018-01).
Of the 5,330 patients included in this study, 5,069 (95.1%) underwent radical mastectomy and 261 (4.9%) underwent breast-conserving surgery (BCS). None of the patients underwent IMN dissection. Moreover, 4,725 patients (88.6%) received adjuvant chemotherapy after surgery. The following three regimens were administered: a classical cyclophosphamide, methotrexate, and fluorouracil regimen; an anthracycline-based regimen; and a combined anthracycline and taxane regimen (henceforth referred to as "taxane-based regimen"). The indications for these three regimens were based on National Comprehensive Cancer Network guidelines. The main indications for radiotherapy included the following: four or more positive lymph nodes in the lymphatic region (selective for patients with one to three positive lymph nodes); primary tumor diameter greater than 5 cm; and BCS. Locoregional radiotherapy was delivered at doses from 46 to 50 Gy. After BCS, all patients received 46 to 50 Gy to the whole breast, followed by a boost of 10 Gy to the primary tumor bed. There were no guidelines defining which patients should receive IMN radiotherapy (IMNRT); radiologists made decisions based on their experience. Adjuvant endocrine therapy was recommended for all patients with estrogen receptor (ER)- or progesterone receptor (PR)-positive tumors. Generally, tamoxifen was administered for 5 years after chemotherapy.
A group of three experienced statisticians performed the statistical analysis. Disease-free survival (DFS) was defined as the interval from the first treatment for breast cancer to the first recurrence (locoregional relapse, distant metastasis, or contralateral breast recurrence). Overall survival (OS) was defined as the period from the date of diagnosis to the date of death from any cause or the date of the last follow-up. Locoregional relapse was defined as the recurrence of cancer in either the treated breast or the ipsilateral lymph node-bearing area (axillary, internal mammary, and supraclavicular nodes). Distant metastasis was defined as metastasis to any other site.
Clinicopathological parameters were assessed using chi-square tests. Cumulative survival probabilities were calculated by the Kaplan-Meier method. Survival rates were compared by log-rank tests. Multivariate analyses were performed using the Cox regression model. Subsequently, variables with a univariate result of p<0.20 [6] and some traditional prognostic factors such as age at diagnosis, tumor size, lymph node involvement, histologic grade, ER/PR status, human epidermal growth factor receptor 2 (HER2) status, and lymphovascular invasion (LVI), were included in the multivariate analysis.
Multivariate analysis was also used to evaluate the prognostic significance of tumor location. To examine whether the effect of IMNRT on survival was related to tumor location, we conducted interaction tests for tumor location (inner vs. outer) and IMNRT (yes vs. no). If an interaction test was significant, indicating that tumor location significantly differed depending on IMNRT, subgroup analyses were performed [7]. Each hazard ratio is presented with a 95% confidence interval. All statistical tests were two-tailed, and p<0.05 was considered significant. Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, USA).
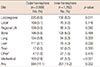
Of the 5,330 patients, 3,568 (66.9%) had tumors located in the outer hemisphere of the breast (upper-outer and lower-outer) and 1,762 (33.1%) had tumors in the inner hemisphere of the breast (upper-inner and lower-inner). Table 1 shows the clinicopathological characteristics of patient subgroups according to breast cancer location. Age at diagnosis, menopausal status, tumor size, pathologic stage, hormone receptor status, HER2 status, LVI, primary surgery, adjuvant radiotherapy, and endocrine therapy were not significantly different between the subgroups. However, significant differences were noted in lymph node status and adjuvant chemotherapy. The outer hemisphere subgroup had a higher percentage of positive lymph nodes, and patients in this subgroup were more likely to receive adjuvant chemotherapy.
The median follow-up time was 68 months (range, 18-176 months). During follow-up, 857 patients (16.1%) relapsed, and 543 patients (10.2%) died from tumor-related causes. The estimated 5-year DFS rates of patients with breast cancer of the outer and inner hemisphere were 81.5% and 77.0%, respectively (p=0.004); the 5-year OS rates were 90.7% and 88.8%, respectively (p<0.001) (Figure 1A, B).
Patients were further categorized by traditional prognostic factors, including age (younger or older than 35 years), lymph node status (negative or positive), tumor size (smaller or larger than 2 cm in diameter), ER/PR status (negative or positive), HER2 status (negative or positive), and LVI status (present or absent). The 5-year DFS and OS rates were not significantly different between patients with breast cancer of the outer versus inner hemisphere, except for patients with positive lymph nodes or tumors lager than 2 cm in diameter. Among the patients with positive lymph nodes, the 5-year DFS rates for patients with breast cancer of the outer and inner hemisphere were 73.1% and 65.8%, respectively (p<0.001); the 5-year OS rates were 86.3% and 83.0%, respectively (p=0.001) (Figure 2A, B). Among the patients with tumors larger than 2 cm in diameter, the 5-year DFS rates for patients with breast cancer of the outer and inner hemisphere were 78.2% and 72.3%, respectively (p=0.002); their OS rates were 89.0% and 86.3%, respectively (p<0.001) (Figure 2C, D).
To explore the role of IMNRT, we analyzed data from 1,201 patients with four or more positive lymph nodes, which was one of the absolute indications for radiotherapy at our institute. Of the 1,201 cases, 288 received IMNRT (212 patients with outer hemisphere lesions and 76 patients with inner hemisphere lesions), while 913 did not receive IMNRT. Traditional prognostic factors were well balanced between the subgroups. The 5-year DFS rates of patients with breast cancer who did or did not receive IMNRT were 69.1% and 54.7%, respectively (p<0.001); their OS rates were 84.3% and 74.1%, respectively (p=0.002) (Figure 3A, B). Among the patients treated with IMNRT, there was no significant difference in the 5-year DFS rate between patients with inner versus outer hemisphere lesions (69.5% vs. 67.1%, p=0.581); similar results were observed for OS (87.2% vs. 86.5%, p=0.678).
Multivariate analysis demonstrated that young age, positive lymph node status, large primary tumor, negative hormone receptor status, HER2 positivity, and presence of LVI were independent adverse prognostic factors for DFS. Similar results were seen for OS (Table 2). After adjusting for the effects of some traditional prognostic factors, tumor location was found to be an independent prognostic factor for DFS and OS. Patients with breast cancer of the inner hemisphere had a higher risk of recurrence and death than patients with breast cancer of the outer hemisphere. Interactions between tumor location and IMNRT were not significant, indicating that the effects of tumor location and IMNRT were independent (Table 2).
Multivariate Cox regression analysis suggested that different prognostic factors applied to tumors in different locations (Table 3). Younger age, positive lymph node status, large tumor size, negative hormone receptor status, positive HER2 status, and LVI were independent unfavorable prognostic factors for DFS in patients with breast cancer of the outer hemisphere. However, only positive lymph node status and large primary tumor size were independent unfavorable prognostic factors for DFS in patients with breast cancer of the inner hemisphere.
Only the location of first recurrence was analyzed in this study (Table 4). A significantly higher incidence of locoregional recurrence-including local sites and regional lymph nodes-and distant organ metastasis-in the lung and liver rather than in the bone, brain, or other distant sites-were observed in patients with breast cancer of the inner hemisphere. Interestingly, patients with breast cancer of the inner hemisphere also had a significantly higher rate of mediastinal lymph node metastasis.
Of the 5,330 patients with operable invasive ductal carcinoma, 33.1% were located in the inner hemisphere and 66.9% were located in the outer hemisphere of the breast. Zucali et al. [1] reported that inner hemisphere tumors were observed in 34.3% of patients. We observed that tumors located in the inner hemisphere were associated with negative lymph nodes more frequently, and patients with these lesions were treated with adjuvant chemotherapy less frequently; this observation is consistent with routine clinical treatment. We also found that patients with inner hemisphere tumors had worse OS than patients with outer hemisphere tumors, and axillary lymph node status was an independent prognostic factor; together, these results suggest that inner hemisphere tumors, which are anatomically closer to the internal mammary lymphatic chain, are more likely to have occult IMN metastases than tumors in nonmedial sites [8]. The other clinicopathological characteristics and treatment types were not significantly different between patients with inner versus outer hemisphere tumors. These results suggest that different tumor locations did not necessarily have different tumor characteristics.
We found that patients with inner hemisphere tumors had significantly worse rates of DFS and OS than patients with outer hemisphere tumors. Differences in survival rates were only observed in patients with positive lymph nodes or large primary tumors (larger than 2 cm in diameter). Most previous studies have suggested that patients with inner hemisphere breast cancer have a higher risk of recurrence and death, but we only observed higher risk of recurrence and death in patients with tumors larger than 2 cm in diameter or pN1 medial lesions [1,8,9,10]. These results seem to indicate at least two main points. First, tumor location itself was an adverse prognostic factor, and not all patients with inner hemisphere tumors had poor prognoses. Second, the risk of IMN involvement increased with tumor size and positive lymph node status [11,12].
The results from our multivariate analysis showed that inner hemisphere tumor location was an important independent prognostic factor for operable breast cancer. After adjusting for all factors linked to prognosis, inner hemisphere tumors were associated with a significantly higher risk of recurrence and death from breast cancer than outer hemisphere tumors (Table 3). Tumor size and lymph node status were the only independent prognostic factors for recurrence of inner hemisphere tumors. Patients with inner hemisphere tumors also had a higher rate of mediastinal lymph node, lung, and liver metastases as the first site of tumor recurrence than patients with outer hemisphere tumors. Notably, mediastinal lymph node metastasis was significantly positively related to lung and liver metastasis. However, the 5-year DFS and OS rates were not significantly different between patients with breast cancer of the outer versus inner hemisphere when IMNRT was performed. This suggests that the increased risk of recurrence and death from breast cancer of patients with inner hemisphere lesions was due to IMN involvement. Understaging and subsequent under-treatment may be the main reasons why patients with inner hemisphere tumors had a higher mortality rate. There is also growing evidence that inner hemisphere tumors are associated with a higher prevalence of positive IMNs [11,13,14,15].
However, studies of surgical or radiological treatment of IMNs have reported conflicting results [3,16,17,18,19]. Several retrospective studies validated the therapeutic efficacy of additional management of IMNs [16,20,21,22]. However, several randomized trials found that there was no survival benefit associated with additional management of IMNs [23,24]. The reasons for these negative results may be lower statistical power and the choice of study population. Assuming that inner hemisphere tumors account for 35% of all cases, and that the incidence of inner hemisphere tumors associated with positive lymph nodes or large tumors is around 50%, we can estimate that a study would need to include 6,100 participants to detect an 8% difference in the survival rate. Therefore, the previous studies had insufficient statistical power. Alternatively, results from the recent European Organization for Research and Treatment of Cancer trial 22922/10925 addressed the impact of IMNRT to a certain extent. In this study, patients with axillary lymph node involvement and/or a medially located primary tumor were randomized to groups that did or did not receive internal mammary and medial supraclavicular lymph node radiotherapy (IM-MS RT). IM-MS RT improved DFS and OS in patients with stage I-III breast cancer, emphasizing the importance of IMNRT in patients with inner hemisphere tumors or axillary lymph node involvement [25].
It is particularly noteworthy that IMNRT may increase the risk of cardiac mortality. Early studies suggested that postoperative irradiation may be associated with an increased incidence of cardiac mortality in patients with left-sided breast cancer [22,26]. However, with the development of modern treatment planning and delivery techniques, the risk of cardiac mortality associated with breast cancer irradiation has declined [27,28], albeit not significantly [23,29]. Unfortunately, data about cardiac events were not recorded in our study.
There were several limitations in this study. This was a retrospective analysis, so our data analysis was limited to the patient and treatment selection biases inherent in such studies. However, given the size of this study, we believe that our results are probably reasonably reliable for the specific patient cohorts that we analyzed. Additionally, exact data for IMN metastasis could not be obtained. We found that the mediastinal lymph nodes were often the site of first recurrence of inner hemisphere tumors. Mediastinal lymph node metastases of breast cancer mainly originate from IMNs, which are the sentinel lymph nodes of the breast. Therefore, mediastinal lymph node metastasis may be a surrogate indicator of IMN metastasis.
In summary, we found that tumor location was a poor prognostic factor for operable breast cancer. IMNRT is recommended for patients with breast cancer of the inner hemisphere with positive axillary lymph nodes or large primary tumors. The role of adjuvant irradiation for IMNs should be further investigated. This is particularly important in the modern era, because radiotherapy now has less short- and long-term toxicities than in the past. Future studies should address the issue of interactions between radiotherapy and concomitant adjuvant systemic treatments.
Figures and Tables
Figure 1
Five-year disease-free survival (DFS) and overall survival (OS) rates by location of breast cancer in different hemispheres (n=5,330). (A) The 5-year DFS. (B) The 5-year OS.

Figure 2
Five-year disease-free survival (DFS) and overall survival (OS) rates for patients who were lymph node positive (n=2,653) or had tumors larger than 2 cm in diameter (n=3,687) by location of breast cancer in different hemispheres. (A) The 5-year DFS for patients who were lymph node positive. (B) The 5-year OS for patients who were lymph node positive. (C) The 5-year DFS for patients who had tumors larger than 2 cm in diameter. (D) The 5-year OS for patients who had tumors larger than 2 cm in diameter.

Figure 3
Five-year disease-free survival (DFS) and overall survival (OS) rates for patients who received additional internal mammary nodes radiotherapy (IMNRT) or not (in those with regional radiotherapy, n=1,201). (A) The 5-year DFS. (B) The 5-year OS.

Table 1
Clinical characteristics and treatment of cancer in different breast hemispheres

Table 2
Multivariate analysis of disease-free survival and overall survival in all population

Table 3
Multivariate analysis of disease-free survival of patients with breast cancer located in different hemispheres

Table 4
Site of first tumor recurrence
References
1. Zucali R, Mariani L, Marubini E, Kenda R, Lozza L, Rilke F, et al. Early breast cancer: evaluation of the prognostic role of the site of the primary tumor. J Clin Oncol. 1998; 16:1363–1366.

2. Arriagada R, Lê MG, Mouriesse H, Fontaine F, Dewar J, Rochard F, et al. Long-term effect of internal mammary chain treatment: results of a multivariate analysis of 1195 patients with operable breast cancer and positive axillary nodes. Radiother Oncol. 1988; 11:213–222.

3. Høst H, Brennhovd IO, Loeb M. Postoperative radiotherapy in breast cancer: long-term results from the Oslo study. Int J Radiat Oncol Biol Phys. 1986; 12:727–732.

4. Lacour J, Bucalossi P, Cacers E, Jacobelli G, Koszarowski T, Le M, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection: five-year results of an international cooperative study. Cancer. 1976; 37:206–214.

5. Xue C, Wang X, Peng R, Shi Y, Qin T, Liu D, et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci. 2012; 103:1679–1687.

6. Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol. 2003; 21:467–472.

7. Colleoni M, Zahrieh D, Gelber RD, Holmberg SB, Mattsson JE, Rudenstam CM, et al. Site of primary tumor has a prognostic role in operable breast cancer: the international breast cancer study group experience. J Clin Oncol. 2005; 23:1390–1400.

8. Lohrisch C, Jackson J, Jones A, Mates D, Olivotto IA. Relationship between tumor location and relapse in 6,781 women with early invasive breast cancer. J Clin Oncol. 2000; 18:2828–2835.

9. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387.

10. Veronesi U, Cascinelli N, Bufalino R, Morabito A, Greco M, Galluzzo D, et al. Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg. 1983; 198:681–684.

11. Shahar KH, Buchholz TA, Delpassand E, Sahin AA, Ross MI, Ames FC, et al. Lower and central tumor location correlates with lymphoscintigraphy drainage to the internal mammary lymph nodes in breast carcinoma. Cancer. 2005; 103:1323–1329.

12. Kong AL, Tereffe W, Hunt KK, Yi M, Kang T, Weatherspoon K, et al. Impact of internal mammary lymph node drainage identified by preoperative lymphoscintigraphy on outcomes in patients with stage I to III breast cancer. Cancer. 2012; 118:6287–6296.

13. Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management. A systematic review. J Clin Oncol. 2008; 26:4981–4989.

14. Chang JS, Park W, Kim YB, Lee IJ, Keum KC, Lee CG, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys. 2013; 86:867–872.

15. Fowble B, Hanlon A, Freedman G, Nicolaou N, Hoffman J, Sigurdson E, et al. Internal mammary node irradiation neither decreases distant metastases nor improves survival in stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 2000; 47:883–894.

16. Stemmer SM, Rizel S, Hardan I, Adamo A, Neumann A, Goffman J, et al. The role of irradiation of the internal mammary lymph nodes in high-risk stage II to IIIA breast cancer patients after high-dose chemotherapy: a prospective sequential nonrandomized study. J Clin Oncol. 2003; 21:2713–2718.

17. Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981; 47:170–175.

18. Olson RA, Woods R, Speers C, Lau J, Lo A, Truong PT, et al. Does the intent to irradiate the internal mammary nodes impact survival in women with breast cancer? A population-based analysis in British Columbia. Int J Radiat Oncol Biol Phys. 2012; 83:e35–e41.
19. Obedian E, Haffty BG. Internal mammary nodal irradiation in conservatively-managed breast cancer patients: is there a benefit? Int J Radiat Oncol Biol Phys. 1999; 44:997–1003.

20. Lê MG, Arriagada R, de Vathaire F, Dewar J, Fontaine F, Lacour J, et al. Can internal mammary chain treatment decrease the risk of death for patients with medial breast cancers and positive axillary lymph nodes? Cancer. 1990; 66:2313–2318.

21. Arriagada R, Rutqvist LE, Mattsson A, Kramar A, Rotstein S. Adequate locoregional treatment for early breast cancer may prevent secondary dissemination. J Clin Oncol. 1995; 13:2869–2878.

22. Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994; 12:447–453.

23. Hennequin C, Bossard N, Servagi-Vernat S, Maingon P, Dubois JB, Datchary J, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013; 86:860–866.

24. Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. 1983; 51:1941–1943.

25. Poortmans P. Irradiation of the internal mammary and medial supraclavicular lymph nodes in stage I to III breast cancer: 10 years results of the EORTC Radiation Oncology and Breast Cancer Groups phase III trial 22922/10925. In : The European Cancer Congress; 2013. p. Abstract BA 2.
26. Paszat LF, Vallis KA, Benk VM, Groome PA, Mackillop WJ, Wielgosz A. A population-based case-cohort study of the risk of myocardial infarction following radiation therapy for breast cancer. Radiother Oncol. 2007; 82:294–300.

27. Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005; 6:557–565.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download