Abstract
Purpose
To date, studies investigating the association between dairy consumption and breast cancer in women have produced conflicting results. As diet is an important, modifiable factor affecting cancer development, the aim of this study was to examine the association between dairy consumption and breast cancer risk.
Methods
PubMed, Embase, and Cochrane Library databases were searched with a priority for prospective cohort studies. Case-control studies were also considered in case of the absence of a cohort study.
Results
We analyzed 22 prospective cohort studies (1,566,940 participants) and five case-control studies (33,372 participants). High and modest dairy consumption (>600 and 400-600 g/day, respectively) significantly reduced the risk of breast cancer compared with low dairy consumption (<400 g/day; risk ratio [RR], 0.90, 95% confidence interval [CI], 0.83-0.98, and RR, 0.94, 95% CI, 0.91-0.98, respectively). A significant linear relationship between dairy consumption and breast cancer risk was found on dose-response analysis. Subgroup analysis found that yogurt (RR, 0.91; 95% CI, 0.83-0.99) and low-fat dairy (RR, 0.85; 95% CI, 0.75-0.96) reduced the risk of breast cancer, while other dairy product types did not. A reduced risk was observed for people in the United States (RR, 0.91; 95% CI, 0.83-0.99) and in those followed for ≥10 years (RR, 0.90; 95% CI, 0.81-0.99). Additionally, the highest level of dairy consumption among Asians was associated with a reduced risk of breast cancer (odds ratio, 0.74; 95% CI, 0.62-0.88).
Breast cancer is the most commonly diagnosed cancer and leading cause of cancer death in women worldwide, accounting for 25.1% (1.67 million) of total new cancer cases and 14.7% (521,907) of total cancer deaths in 2012 [1]. Age-standardized incidence and mortality rates are between 19.3-89.9 and 6.3-17.5 per 100,000 population, respectively [1]. Reproductive and hormonal factors, age, physical inactivity, obesity, and alcohol consumption have been considered risk factors associated with the increasing incidence of breast cancer [23]. Recently, the relationship between lifestyle, dietary factors and the risk of breast cancer have been extensively studied because diet is considered one of the modifiable risk factors for breast cancer.
The risk of breast cancer is affected both negatively and positively by dairy products through several potential mechanisms. The main hypothesis proposing that dairy consumption decreases breast cancer risk cites the anticarcinogenic properties of calcium, vitamin D, butyrate, lactoferrin, and conjugated linoleic acid [45]. Dairy products are the main dietary sources of these compounds, and studies have suggested that they play a role in reducing breast cancer risk [67]. However, it has also been suggested that dairy products increase breast cancer risk. High dairy product consumption may reflect an overall higher dietary fat intake, particularly saturated fat, which in turn has been associated with higher breast cancer incidence. Milk also contains various factors that may affect human health, for example, insulin-like growth factor I (IGF-I), which has been shown to promote breast cancer cell growth, estrogen, which is associated with increased mitotic activity and DNA replication errors, and various contaminants, such as potentially carcinogenic pesticides [89].
Although the incidence rate for female breast cancer in the majority of Asian countries is much lower than that in Western countries, there has been a marked increase in recent years [1]. Furthermore, there are also etiological differences in breast cancer between the East and West. Thus, there is a need to explore the true relationship between dairy consumption and the risk of breast cancer in different populations.
To date, the results of various prospective studies and meta-analyses examining associations between dairy product consumption and the risk of breast cancer have been inconsistent. Differences in the outcomes of these studies may be partly due to discrepancies in dairy consumption between the different studies. Moreover, these studies have mainly been conducted in the United States or Europe, with only one Asian cohort from Japan. With so few prospective Asian studies available for analysis, we suggest that where available, case-control studies should be assessed instead.
In summary, the effect of different populations, differing types of dairy products, and possible dose-response relationships on breast cancer risk remains unknown. The objective of this study was therefore to conduct a dose-response meta-analysis and summarize the epidemiological evidence for any relationship between dairy consumption and risk of breast cancer. This is important because estimating breast cancer risk associated with dairy consumption may help to inform decision-making for physicians and public health policy.
Relevant studies published until January 2014 were identified by searching PubMed, Embase, and The Cochrane Library, without language restrictions. We searched using the following terms: "dairy products", "dairy", or "milk", in combination with the terms: "breast cancer" or "breast neoplasms". This search was supplemented by a manual search of the 1994 to 2013 Annual Meeting Proceedings of the American Society of Clinical Oncology. Relevant reviews and meta-analyses on the role of dairy intake on the risk of breast cancer were examined for potential inclusive studies. This study was performed according to the guidelines described in the Meta-Analysis of Observational Studies in Epidemiology [10].
Citations selected from the initial search were subsequently screened for eligibility. For inclusion, studies were required to (1) have examined the relationship between dairy product consumption (including any type of milk, yogurt, cheese, cottage cheese, and other dairy products) and the incidence risk of breast cancer (prospective studies) or the odds of breast cancer (case-control studies); (2) be prospective in nature, except for studies from Asian countries; and (3) include sufficient data for different dosage categories of dairy consumption, including relative risk (RR) or odds ratio (OR), with corresponding 95% confidence interval (CI).
We firstly divided the consumption of dairy into three groups: high (>600 g/day), modest (400-600 g/day), and low (<400 g/day). Then we compared the RR between high and low/modest consumption. We also examined the relationship between breast cancer risk and consumption of different dairy types, population, geographic area, and follow-up duration.
Dairy consumption was assessed in the original studies using either a food frequency questionnaire or a 24-hour recall data interview. Dairy items (e.g., milk, cheese, and butter) were converted into milk servings according to the protein content.
Data abstraction and quality assessment were conducted independently by two reviewers (J.Z. and M.S.) using a standardized approach. A third reviewer (S.Z.) adjudicated disagreements after referring to the original articles. Data retrieved from the reports included publication details, methodological components, and the following trial characteristics: title, author, publication year, country in which study was conducted, sample size, age of subjects, follow-up duration, and covariates controlled for by matching or multivariate analysis. The number of cases/noncases or person-year data and adjusted RR/OR for each consumption category (together with corresponding 95% CI) were extracted or estimated. For studies that reported several multivariate adjusted RRs/ORs, the effect estimate was extracted after fully adjusting for potential confounders. Study quality was assessed using the 9-star Newcastle-Ottawa Scale by two investigators (J.Z. and M.S.) [11].
We examined the relationship between dairy consumption and the risk of breast cancer on the basis of the adjusted RRs/ORs and 95% CIs reported in each study. A fixed-effect model was used to estimate the pooled RRs/ORs, with 95% CIs if there was no evidence of heterogeneity; otherwise, a random-effect model was adopted.
A dose-response meta-analysis was then conducted from the correlated natural log of the RRs across the categories of dairy consumption [12]. Dairy intakes were converted from servings or other units into grams per day (g/day) using standard conversions from the Food Standards Agency guidelines [13] (1 serving=200 g; 1 cup =237 g; 1 glass=200 g), or by using the average intake in each quartile from the studies most similar to one another [13]. To derive the dose-response curve, dairy consumption was modeled either using the linear model or using restricted cubic splines with three knots at fixed percentiles (10%, 50%, and 90%) of the distribution. The p-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. The details of the methods used have been described elsewhere [1415].
Statistical heterogeneity among studies was evaluated using the χ2 test and I2 statistic. The Egger's regression test [16], Begg's test [17], and visual inspection of a funnel plot were performed to assess publication bias. Subgroup analyses by geographic area, years of follow-up, type of dairy product, and menopause status were performed to examine the relationship between dairy consumption and breast cancer risk. Sensitivity analyses were performed in two ways: first, by excluding those studies that met relatively fewer quality criteria of the Newcastle-Ottawa Scale (<7 stars), and second, by excluding the studies individually to investigate the influence of a single study on the overall risk estimation.
Stata V.12.0 software (Stata, College Station, USA) was used for all analyses, and all statistical tests were two-sided; p<0.05 was considered an indication of statistical significance.
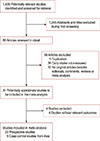
A flow diagram of our literature search was illustrated in Figure 1. As of January 2014, 1,435 records were retrieved using the search strategy described in the methods section. After reviewing the titles and abstracts, 89 articles met the inclusion criteria for the meta-analysis. Fifty-eight articles were excluded for the following reasons: articles were not original studies (editorials, comments, reviews, or meta-analyses), dairy intake was not measured, duplication of reports from the same study populations, and no data on breast cancer. In total, 27 studies were included in this meta-analysis. Twenty-two prospective studies assessing the relationship between dairy or milk intake and the risk of breast cancer were evaluated in the study. Of these, 13 examined the effect of dairy and 16 examined the effect of milk intake on breast cancer incidence. Five studies provided data on childhood dairy consumption, five examined both fermented milk and yogurt consumption, and six and five studies evaluated whole milk and skim milk consumption, respectively. Low- and high-fat dairy consumption were analyzed in four and three studies, respectively, whilst seven studies provided data on cheese intake. Five case-control studies conducted in Asia were also included in the analysis: two from Japan, two from China, and one from Iran. Two of these studies reported on milk intake and three on dairy intake.
Table 1 lists the general characteristics of the 22 prospective (1,566,940 participants and 37,925 breast cancer cases) and five case-control (33,372 participants and 7,418 breast cancer cases) studies we included in the analysis. The prospective cohort studies were published between 1989 and 2013: 11 were conducted in the United States, 10 in Europe, and one in Japan. The majority of the studies were population-based, but three were conducted only in nurses. Three studies were conducted among premenopausal women only, three among postmenopausal women only, and six presented results by menopausal status. The length of follow-up ranged from 4 to 65 years, with a median of 10 years. Assessment of dairy intake was not consistent between studies, determined through either diet questionnaires or structured food frequency questionnaires to gather data. Case ascertainment also differed between studies, with most using medical records, whilst some used self-reports, of which the majority were confirmed by medical records. Adjustment for potential confounding factors differed across studies and most risk estimates were adjusted for age, body mass index, family history of breast cancer, reproductive factors, hormone replacement therapy, and total energy intake.
The summary of RR of breast cancer for high (>600 g/day) compared with low (<200 g/day) dairy product consumption and dairy subgroups is shown in Figure 2. High-level dairy consumption was associated with a statistically significantly lower risk of breast cancer (RR, 0.90; 95% CI, 0.83-0.98; I2=32.2%; p-value for heterogeneity=0.111). The random effect model was adopted. Modest dairy consumption (400-600 g/day) was associated with a mildly lower risk of breast cancer (RR, 0.94; 95% CI, 0.91-0.98; I2=0%; p-value for heterogeneity=0.975).
A dose-response analysis revealed evidence of a linear relationship between dairy consumption and risk of breast cancer (p=0.016) (Figure 3). Compared with no dairy consumption, the adjusted RRs were 0.97 (95% CI, 0.95-0.99) for 250 g/day, 0.94 (95% CI, 0.89-0.99) for 500 g/day, 0.91 (95% CI, 0.85-0.98) for 750 g/day, and 0.88 (95% CI, 0.80-0.98) for 1,000 g/day.
High milk consumption was not found to have a preventive effect on breast cancer compared to low milk consumption (RR, 0.94; 95% CI, 0.86-1.03; I2=45.8; p-value for heterogeneity=0.018). As shown in Figure 4, no evidence of a linear or nonlinear relationship between milk consumption and risk of breast cancer was found.
Subgroup analyses were stratified by dairy type, study population, geographic area, and follow-up period (Figure 2). We found that consumption of yogurt and low-fat dairy were associated with a significant reduction in the risk of breast cancer (RR yogurt, 0.91, 95% CI, 0.83-0.99, I2=0.0%, p-value for heterogeneity=0.991; RR low fat dairy, 0.85, 95% CI, 0.75-0.96, I2=42.7%, p-value for heterogeneity=0.121). However, no significant reduction was observed with whole milk, skim milk, cheese/butter, or high-fat dairy consumption. Considering population demographics, there was a marginally reduced breast cancer risk in premenopausal women (RR, 0.88, 95% CI, 0.77-1.00, p=0.057; I2=0%, p-value for heterogeneity=0.425). No association was found between breast cancer risk and postmenopausal women or childhood dairy consumption. Focusing on the study country, those evaluating the U.S. population demonstrated a reduced risk of breast cancer (RR, 0.91, 95% CI, 0.83-0.99; I2=36.5%, p-value for heterogeneity=0.126). However, no statistically significant reduction in risk was observed among Europeans (RR, 0.96, 95% CI, 0.82-1.12; I2=48.1%, p-value for heterogeneity=0.103). Examining follow-up duration, we identified a significantly reduced risk of breast cancer when subjects were followed for ≥10 years (RR, 0.90, 95% CI, 0.81-0.99; I2=58.4%, p-value for heterogeneity=0.034) (Figure 2).
The summary of OR of breast cancer for high-level compared with low-level dairy or milk product consumption is illustrated in Figure 5A. A high level of dairy or milk consumption was associated with a significantly lower risk of breast cancer (OR, 0.74, 95% CI, 0.62-0.88; I2=62.5%, p-value for heterogeneity=0.014). The random effect model was adopted. Evidence of a linear relationship between dairy or milk consumption and risk of breast cancer was found in the dose-response analysis (p=0.002) (Figure 5B). Compared with that for no dairy or milk consumption, the adjusted OR was 0.85 (95% CI, 0.76-0.94) for 250 g/day, 0.71 (95% CI, 0.58-0.88) for 500 g/day, 0.60 (95% CI, 0.44-0.83) for 750 g/day, and 0.51 (95% CI, 0.33-0.78) for 1,000 g/day of dairy product consumption.
The results of a sensitivity analysis performed after excluding studies of low quality were similar to the original results (RR dairy, 0.89, 95% CI, 0.82-0.98; RR milk, 0.93, 95% CI, 0.85-1.02). A sensitivity analysis investigating the influence of a single study on the overall risk estimate by omitting one study in turn suggested that overall risk estimates were not substantially modified by any single study, with a range from 0.89 (95% CI, 0.82-0.97) to 0.93 (95% CI, 0.87-0.98) for total dairy food intake and from 0.93 (95% CI, 0.85-1.02) to 0.96 (95% CI, 0.89-1.04) for milk intake. Excluding the single Asian prospective study (Japan) did not significantly change the results. Moreover, when this cohort was added to the analysis of case-control studies from Asia, the results remained consistent.
The funnel plot and Egger's test identified no significant publication bias in the comparison between highest and lowest or modest and lowest dairy consumers, or the highest and lowest milk consumers (Figure 6). Similarly, the results of case-control studies showed no publication bias.
The findings of this meta-analysis, which included more than 1.6 million participants, indicated that dairy consumption is inversely and significantly associated with the development of breast cancer. A dose-response analysis also confirmed this inverse association, demonstrating a significant linear relationship between the amount of dairy consumed and breast cancer risk. Subgroup analyses suggested inverse associations between breast cancer risk and consumption of yogurt, consumption of low-fat dairy products, women from America, and a follow-up period of >10 years. The case-control studies from Asia indicated that higher dairy or milk consumption was associated with a 27% reduction in breast cancer risk. Diet is considered a modifiable risk factor for breast cancer. These findings may therefore help to inform decision-making in public health policy.
Several compounds present in dairy products may be responsible for the observed association between dairy consumption and breast cancer risk. Data from in vitro studies suggest that calcium and vitamin D exert anticarcinogenic effects on breast cancer cells [1819]. Observational studies and meta-analyses have also provided evidence that vitamin D and calcium protect against breast cancer, especially in premenopausal women [2021]. In some countries, such as the United States, but not in most European countries, dairy products are fortified with vitamin D. This makes any association between dairy consumption, vitamin D levels, and breast cancer more difficult to distinguish. One of our findings from the subgroup analysis showed dairy consumption in the United States to be significantly associated with a lower risk of breast cancer; this association was not found in Europeans. The discrepancy in vitamin D fortification of dairy products between different countries may have contributed to these differing results.
In contrast, various other compounds present in dairy products, such as saturated fatty acids, endogenous IGF-I and other potential contaminants, are considered to be potentially carcinogenic. Dietary fat intake has long been considered to increase the incidence of breast cancer [2223]; however, it is thought that different types of fatty acids may contribute differently to breast cancer risk. Whilst saturated fatty acids have been associated with increased breast cancer risk, no significant association has been demonstrated for total, monounsaturated, or polyunsaturated fats [2425]. By subgroup analysis, we found that low-fat, but not high-fat dairy consumption reduced the risk of breast cancer. Low-fat dairy differs from high-fat dairy in the content of the different types of fatty acids present and is produced by filtering full-fat dairy to remove the majority of saturated fatty acids, while retaining unsaturated fatty acids. This may explain the different and conflicting results regarding the effect of fat intake on the risk of breast cancer in different studies.
Subgroup analysis also showed that fermented dairy or yogurt consumption was associated with a lower breast cancer risk. Yogurt is nutritionally rich in probiotics, protein, calcium, riboflavin, vitamin B6, and vitamin B12, and has nutritional benefits beyond those of milk [20]. Lactobacillus acidophilus, a probiotic present in yogurt, may modulate the immune response against breast cancer in a murine model. With increasing age, intestines exhibit declining levels of bifidus bacteria, which allows the growth of toxin-producing and, perhaps, cancer-inducing bacteria. Yogurt can provide probiotics to replenish and balance the microflora in the intestines, which may lower cancer risk [26]. Further, IGF-I content, which may increase the risk of breast cancer, is significantly reduced in processed dairy products by heat treatment or fermentation [27]. These factors may therefore contribute to the observed reduction in breast cancer risk in those who consume fermented dairy or yogurt.
We believe that the results of this study are more reliable than those recently presented in the systematic review by Dong et al. [28]. Although Dong et al. also showed an inverse association between dairy consumption and breast cancer risk, they only compared the highest level of dairy intake versus the lowest level to point synthesis, instead of using a dose-response analysis, which should provide a more reliable result. Moreover, several large cohort studies were not included in their study, but are included here. Excluding these studies may have resulted in insufficient statistical power and publication bias. Further, the studies they included were largely from the United States and Europe. The relationship between dairy and breast cancer risk in Asian populations, which accounts for almost one-third of the world's population, was not examined. Although we found few prospective studies conducted in Asia, we searched case-control studies to assess the relationship between dairy consumption and breast cancer risk. Our results also demonstrated a significant protective effect of dairy on breast cancer risk for Asian populations. Genetic factors associated with breast cancer development in Asians differ from those in Western women. Additionally, dietary patterns are different, with a lower dairy consumption among Asian compared to Western populations. It is possible that the effect of dairy consumption is overestimated in Asian populations because of the lower consumption. These results also suggest differing etiologies for breast cancer development in different populations.
This meta-analysis has several strengths, which we believe increased the reliability and validity of the findings. First, prospective studies from the United States and Europe were included, providing lower recall bias and selection bias. Second, we assessed the relationship between dairy intake and breast cancer risk based on geographic area, which is important given the observed effect in Asian populations. Third, the dose-response analysis included a wide range of dairy consumption levels, which allowed an accurate assessment of the dose-response relationship between dairy consumption and breast cancer risk.
There were several possible limitations in this meta-analysis, which must be considered when interpreting the results. First, although major potential confounders had been adjusted for in most of the studies that we included, residual or unknown confounders cannot be excluded. Meta-analysis is unable to resolve problems with confounding factors that could be inherent in the original study design. High-dairy, low-fat dairy, or yogurt consumers may share, or be exposed to, a greater number of beneficial environmental factors compared with low-dairy or other types of dairy consumers, such as good economic status, better educational opportunities, a greater ability to engage in physical activity, and access to a generally healthier lifestyle. Breast cancer is believed to result from the interactions between both environmental and genetic factors [2930], but few data regarding genetic factors were contained in the primary aggregative results. A second limitation was the misclassification of dairy consumption, which was inevitable given that consumption of dairy was self-reported in most studies and only a few studies updated information on dairy consumption during follow-up. The different dairy classifications may have been introduced because dietary assessments were based on different questionnaires and different nutrient databases. Misreporting of high dairy consumption or changes in consumption during follow-up would most likely lead to an incorrect estimate of the true association between dairy consumption and risk of breast cancer. Thirdly, heterogeneity may have been introduced because of methodological differences between studies. However, in this meta-analysis, no significant evidence of heterogeneity was found. The sensitivity analyses regarding methodological differences have also yielded consistent results with those in the overall analysis. Finally, the use of case-control studies from Asia may provide a lower level of evidence compared to the prospective studies, but these were included in the analysis because there were too few prospective Asian studies available. A greater effect of dairy consumption on breast cancer risk was observed in these Asian studies compared to that found in the studies from United States and Europe. We propose that further prospective studies should be conducted to reveal the true relationship between dairy and breast cancer amongst Asians.
In conclusion, dairy consumption was inversely associated with the risk of breast cancer in a manner that appears to be dose-dependent, time-dependent, and dairy-type dependent in the United States and Europe. A large effect was also observed for Asian case-control studies. Prospective studies that investigate gene-environment interactions are required to further clarify the etiology of breast cancer.
Figures and Tables
 | Figure 2Combined relative risks (RRs) of the breast cancer for dairy, milk consumption and main subgroups (highest dairy consumption was deemed as >600 g/day; modest, 400-600 g/day).CI=confidence interval.
|
 | Figure 5The summary odds ratio (OR) of breast cancer for high level of dairy or milk product compared with low level consumption (A) and doseresponse analysis for case-control studies from Asia (B).CI=confidence interval; POM=postmenopausal; PRM=premenopausal; RR=relative risk.
|
 | Figure 6Funnel plot of log relative risk (RR) versus standard error (s.e.) of log RRs for highest dairy versus lowest dairy consumption (A); for modest dairy versus lowest dairy consumption (B); for highest milk versus lowest dairy consumption (C); for case-control study (D). |
Table 1
The characteristics of included studies

Notes
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2008; 61:203–213.

3. Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007; 8:292–293.

4. Białek A, Tokarz A. Conjugated linoleic acid as a potential protective factor in prevention of breast cancer. Postepy Hig Med Dosw (Online). 2013; 67:6–14.

5. Davoodi H, Esmaeili S, Mortazavian AM. Effects of milk and milk products consumption on cancer: a review. Compr Rev Food Sci Food Saf. 2013; 12:249–264.

7. Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002; 94:1301–1311.

8. Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003; 12:739–746.
9. Mattisson I, Wirfält E, Johansson U, Gullberg B, Olsson H, Berglund G. Intakes of plant foods, fibre and fat and risk of breast cancer: a prospective study in the Malmö Diet and Cancer cohort. Br J Cancer. 2004; 90:122–127.

10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.

11. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute;Accessed April 20th, 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
12. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006; 6:40–57.

13. Crawley H, Mills A, Patel S. Food Standards Agency. Food Portion Sizes. 3rd ed. London: TSO;2002.
14. Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012; 95:362–366.

15. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012; 175:66–73.

16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

18. Xie SP, James SY, Colston KW. Vitamin D derivatives inhibit the mitogenic effects of IGF-I on MCF-7 human breast cancer cells. J Endocrinol. 1997; 154:495–504.

19. Xie SP, Pirianov G, Colston KW. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. Eur J Cancer. 1999; 35:1717–1723.

20. Redaniel MT, Gardner MP, Martin RM, Jeffreys M. The association of vitamin D supplementation with the risk of cancer in postmenopausal women. Cancer Causes Control. 2014; 25:267–271.

21. Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. J Womens Health (Larchmt). 2013; 22:915–929.

22. Lamas B, Nachat-Kappes R, Goncalves-Mendes N, Mishellany F, Rossary A, Vasson MP, et al. Dietary fat without body weight gain increases in vivo MCF-7 human breast cancer cell growth and decreases natural killer cell cytotoxicity. Mol Carcinog. 2015; 54:58–71.

23. Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: an evolutionary perspective. Am J Hum Biol. 2011; 23:601–608.

24. Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiébaut AC, et al. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008; 88:1304–1312.

25. Schulz M, Hoffmann K, Weikert C, Nöthlings U, Schulze MB, Boeing H. Identification of a dietary pattern characterized by high-fat food choices associated with increased risk of breast cancer: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br J Nutr. 2008; 100:942–946.

26. Merenstein DJ, Smith KH, Scriven M, Roberts RF, Sanders ME, Petterson S. The study to investigate the potential benefits of probiotics in yogurt, a patient-oriented, double-blind, cluster-randomised, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010; 64:685–691.

27. Kang SH, Kim JU, Imm JY, Oh S, Kim SH. The effects of dairy processes and storage on insulin-like growth factor-I (IGF-I) content in milk and in model IGF-I-fortified dairy products. J Dairy Sci. 2006; 89:402–409.

28. Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011; 127:23–31.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download