Abstract
Breast cancer coexisting with lymphoma is a rare condition with various diagnostic and therapeutic implications. In this report, we describe the case of a 55-year-old Caucasian woman who presented with simultaneous ductal carcinoma in situ of the right breast, and follicular lymphoma involving an inguinal lymph node and the left breast. The patient underwent local excision and radiotherapy for the ductal carcinoma in situ, while a watch and wait strategy was adopted for the lymphoma. Two years later, the patient presented with multifocal ductal carcinoma of the left breast and reappearance of the lymphoma in the left axillary lymph nodes. She underwent bilateral mastectomy, left sentinel node biopsy, and chemotherapy. Synchronous follicular lymphoma and bilateral metachronous breast carcinoma has not been described previously. Diagnosis is based on tissue histology after excision or a needle biopsy. Treatment for these two diseases is distinct, and a multidisciplinary approach should be adopted.
Synchronous breast cancer and follicular lymphoma of the breast is a rare condition [1234]. The differential diagnosis between breast lymphoma and breast adenocarcinoma is difficult clinically and radiologically as these diseases share common characteristics [5], and the diagnosis is usually confirmed postoperatively.
Diagnosis of the two conditions in a single patient is often thought to be coincidental, but there is evidence supporting a possible pathophysiological connection involving a common genetic background or etiologic factor, or possibly, one disease causes the other [6]. In this case, the presentation of multifocal bilateral epithelial breast cancer with three primary tumors suggests that tumorigenesis was not coincidental.
It has also been suggested that breast lymphoma can change the behavior of concomitant breast cancer either by altering the axillary lymphatic vessels, which aids breast cancer dissemination [3], or by changing the lymph node microenvironment [4].
Distinct treatment strategies are required for these two diseases including surgery, radiotherapy, hormonal therapy, chemotherapy, and immune therapy, depending on the disease type and patient characteristics such as stage of disease, aggressiveness, histological subtype, and estrogen receptor (ER)/human epidermal growth factor receptor 2 (HER2) status [5].
Here, we report the unique case of a patient with ductal carcinoma in situ (DCIS) and follicular lymphoma of the breast, who also presented with second primary contralateral multifocal breast cancer at a later date.
A 55-year-old Caucasian woman presented in April 2011 to the hospital with a painless lump in the upper outer quadrant of the left breast, discovered during a breast self-examination. A second painless nodule was reported in the right inguinal area. She was otherwise asymptomatic.
The patient had a 20-30 pack-year smoking history and had one full-term pregnancy previously. Her medical history was significant for a swelling of the left parotid gland 6 months before her current presentation. This was investigated by ultrasonography and fine-needle aspiration (FNA), which revealed an enlarged benign intraparotid lymph node. There was no family history of lymphoma, breast, or ovarian cancer.
A clinical examination revealed a small, firm, painless, ill-defined nodule in the upper outer quadrant of the left breast with no associated lymphadenopathy. An enlarged lymph node was found in the right inguinal area, which corresponded to the nodule detected by the patient. The examination of the right breast and axilla, neck, abdomen, and left inguinal area was unremarkable.
A bilateral mammography and breast ultrasound revealed a single lesion in the upper outer quadrant of each breast with characteristics suspect of malignancy, but a tissue diagnosis was warranted (Figure 1). The patient underwent excision biopsy of both breast lesions and the inguinal nodule.
A histological examination of the left breast specimen revealed the presence of a lymph node with complete coagulative necrosis. The necrotic area contained some medium-sized shadow cells, which were immunohistochemically positive for cluster of differentiation 20 (CD20) and CD10, and were admixed with some small B-cells. Dense, diffuse, periductal lymphoid infiltration was noted around the necrosis. Immunohistochemical analysis showed B- and T-cells, and scattered cells positive for CD10, CD23, and B-cell CLL/lymphoma 6 (Bcl-6) (Figure 2).
A histological examination of the right breast specimen revealed a typical DCIS, which followed an ethmoid pattern with intermediate nuclear-grade and comedo-type necrosis (Figure 3).
Accordingly, histological analysis showed that the inguinal lymph node was infiltrated by a follicular B-cell lymphoma grade 1/2 (Figure 4). The neoplastic cells were arranged in a predominantly diffuse and partly nodular pattern. The number of centroblasts was <15/high-power field. Immunohistochemical analysis showed that the cells were positive for CD10, Bcl-6, and Bcl-2, and partly for CD23. The tumor Ki-67 proliferative index was low at approximately 10%. Cells positive for CD10 and Bcl-6 were found in the interfollicular area, and monoclonality for the kappa light chain was also noted. The neoplastic cells had infiltrated the nodal capsule and spread to the perinodal adipose tissue.
The patient underwent computed tomography of the cervix, thorax, and abdomen, which was normal. Full blood counts and blood biochemistry test results were also normal. Slightly elevated polyclonal IgM levels were found. A bone marrow biopsy was negative for lymphomatous infiltration.
The final diagnosis was simultaneous DCIS of the right breast and non-Hodgkin B-cell follicular lymphoma, stage IIIEA. Right breast irradiation was administered for DCIS postoperatively. As the follicular lymphoma was disseminated, but did not fulfill the criteria for treatment initiation, a watch and wait strategy was adopted.
In December 2013, a routine follow-up mammography showed two speculated lesions in the left breast. One was retroareolar and measured <1 cm in maximum diameter, while the other lay deeper and measured approximately 1.5 cm in diameter. Ultrasonography and magnetic resonance imaging confirmed the suspicious findings, and an ultrasound-guided core biopsy was taken from the largest lesion. This revealed a grade II invasive ductal carcinoma (IDC), positive for the ER/ progesterone receptor (PR) and HER2 (2+). New staging investigations revealed no evidence of lymphoma and no metastases from the breast disease.
After several consultations with the patient, a bilateral routine skin-reducing mastectomy with an inferior dermal flap and silicon-based reconstruction, together with a left sentinel node biopsy was performed.
The breast histopathologic examination revealed 2 grade II IDCs, measuring 0.9 cm and 1.4 cm with surrounding DCIS. ER and PR were strongly positive, and fluorescence in situ hybridization analysis confirmed HER2 amplification. The sentinel lymph nodes were free of breast cancer deposits. However, immunohistochemical analysis for CD20, CD3, CD10, bc1-2, and Ki-67 was indicative of in situ follicular lymphoma as shown by the presence of lymphoma cells Bcl-2 (+), Bcl-6 (+), CD10 (+) confined into the germinal center (Figure 5).
Subsequently, the patient completed four cycles each of anthracycline and taxane-based chemotherapy in September 2014, and was prescribed trastuzumab in combination with an aromatase inhibitor. The patient resumed routine follow up, and a genetic consultation was encouraged.
Synchronous breast cancer and follicular lymphoma is a rare clinical condition, and has been described only a few times in the literature. Our case describes the synchronous presentation of DCIS and secondary breast lymphoma (according to the Wiseman and Liao criteria [7]), further complicated by metachronous contralateral infiltrating breast carcinoma.
Primary or secondary breast lymphoma may have a clinical presentation indistinguishable from breast cancer as it usually manifests as a breast lump or axillary lymphadenopathy [1]. Mammographic imaging can also be confusing as it shows a speculated mass or microcalcifications [5]. Generally, the diagnosis of lymphoma is made postoperatively after the histological examination, but occasionally, analysis of the preoperative FNA or core biopsy can lead to a diagnosis.
Breast cancer patients have been found to have an increased incidence of non-Hodgkin lymphoma because of treatment with radiotherapy and chemotherapy [2]; however, this does not explain cases with synchronous disease. There are several hypotheses regarding the connection between these two entities, including the role of epidemiologic factors, such as age or gender, or a common genetic background, or possibly that one disease causes the other.
With regard to the common genetic background, mutations in the ataxia telangiectasia mutated gene have been found in both lymphoid neoplasia and breast cancer [2]. Furthermore, the mouse mammary tumor virus is strongly suspected to play an etiologic role in both tumors, as its genetic sequence or similar sequences have been discovered in both breast cancer and lymphoma cells, but not in nonneoplastic cells [3]. However, these data are controversial. Finally, results from rare cases of collision tumors support the hypothesis that breast cancer may act as an antigenic stimulus and cause non-Hodgkin lymphoma [4], analogous to cases where Helicobacter pylori inflammation of the stomach may cause mucosa-associated lymphoid tissue lymphoma.
Furthermore, it seems that the connection between these two diseases may not just be etiologic and there may be synergism between the two cancers after tumorigenesis has occurred. One important theory is that the lymphoma could obliterate the lymphatic channels, thus altering the predominant mode of breast cancer metastasis. There are also clinical data suggesting that lymphomas can change the course of a radioactive tracker, diminishing the utility of a sentinel lymph node biopsy to indicate breast cancer lymphatic dissemination [6]. Moreover, neoplastic lymphoid cells could reduce tissue necrosis factor or interleukin-induced adhesion of breast cancer cells to the endothelial layer of the axillary lymph nodes, thus facilitating lymphatic dissemination to certain lymph nodes [5]. However, since some lymphomas develop outside of the nodal sinuses, these theories are not universally accepted, and any interaction between breast cancer and breast lymphoma may be overestimated [8].
Only six similar reports were published previously [12349]. Barranger et al. [4] described a case of T1N0 IDC coexisting with lymphoma in the ipsilateral axillary lymph nodes. Cox et al. [3] described two cases, one with T1N0 IDC coexisting with disseminated follicular lymphoma (stage III) and one with DCIS coexisting with lymphoma in the ipsilateral axillary lymph nodes. Cuff et al. [2] reported a case of synchronous invasive lobular carcinoma and follicular lymphoma discovered during sentinel lymph node dissection. Laudenschlager et al. [1] describe a case of T3N0M0 IDC coexisting with follicular lymphoma in the ipsilateral lymph nodes. Finally, Tamaoki et al. [8] described DCIS of the breast coexisting with lymphoma in the para-aortic lymph nodes. In all of these reports, lymphoma was discovered incidentally during the histologic examination of the specimen; however, in our case, a palpable inguinal lymph node was present.
The occurrence of metachronous breast tumor after the synchronous diagnosis of DCIS and lymphoma is unique. While lymphoma patients have a higher risk for second tumor development due to the therapy they have received, this does not apply to our patient as she did not receive any treatment. To our knowledge, only two similar cases involving three primary malignancies have been described in the literature. The coexistence of DCIS in the right breast and diffuse large B-cell lymphoma in the right axilla, and invasive ductal carcinoma in the left breast has been described by Miles and Jacimore [10], while synchronous bilateral invasive ductal carcinoma and primary breast lymphoma has been described by Garg et al. [11].
In conclusion, the coexistence of breast cancer and follicular breast lymphoma, complicated by contralateral metachronous multifocal mammary carcinoma has not been described before now in the literature. There appears to be a pathophysiol ogical connection between the two diseases, involving cancer pathogenesis and behavior, although this may be due to circumstantial evidence. Management of coexisting breast lymphoma and breast cancer may be a challenge, requiring input from surgeons, medical and clinical oncologists, radiologists, and pathologists. A multidisciplinary approach, a high level of clinical vigilance, and teamwork are required to avoid an undesirable outcome.
Figures and Tables
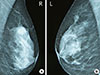 | Figure 1Bilateral mammography of the patient. Both breasts are heterogeneously dense. (A) Right: There is a high-density irregular mass in the upper outer quadrant of the breast. It has indistinct margins, possibly spiculated at places but without significant architectural distortion. The mammogram was graded as Breast Imaging Reporting and Data System (BI-RADS) category 4. (B) Left: There is an asymmetric density with microcalcifications in the upper outer quadrant of the breast corresponding to the palpable lump. The finding has ill-defined or microlobulated margins at places and is not associated with architectural distortion. The mammogram was graded as BI-RADS category 4. |
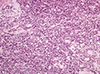 | Figure 2Microscopic finding of secondary follicular lymphoma involving left breast. Neoplastic lymphoid cells, mainly centrocytes with a few scattered centroblasts, diffusely infiltrating breast parenchyma (H&E stain, × 200). |
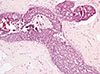 | Figure 3Microscopic findings of right breast ductal carcinoma in situ (DCIS). Low grade DCIS of cribriform pattern with the presence comedo necrosis involving two ducts (H&E stain, ×200). |
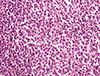 | Figure 4Microscopic findings of inguinal lymph node specimen. Neoplastic lymphoid cells consisting of centrocytes and some few centroblasts (<15/high-power field) infiltrated the inguinal lymph node, thus disturbing the lymph node normal architecture. The neoplastic populating is arranges in a predominantly nodular pattern (H&E stain, ×200). |
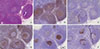 | Figure 5Histological specimen of sentinel lymph node after second excision. (A) Histological examination of in situ follicular lymphoma in sentinel lymph node after second excision (H&E stain, ×100). (B) CD20 immunopositivity showing the nodules corresponding to infiltrated germinal centers (×100). (C) CD3 immunohistochemical staining showing the reactive T cells arranged mainly outside the nodules (×100). (D) Bcl-2 immunopositivity in the lymphoma B cells inside the germinal centers (×100). (E) CD10 immunopositivity in the neoplastic nodules excluding the probability of being primary follicles, the latter being CD10 negative (×100). (F) Ki-67 index in the neoplastic nodules which is rather low, compared to the expected increased one in reactive germinal centers (×100). |
References
1. Laudenschlager MD, Tyler KL, Geis MC, Koch MR, Graham DB. A rare case of synchronous invasive ductal carcinoma of the breast and follicular lymphoma. S D Med. 2010; 63:123–125.
2. Cuff KE, Dettrick AJ, Chern B. Synchronous breast cancer and lymphoma: a case series and a review of the literature. J Clin Pathol. 2010; 63:555–557.

3. Cox J, Lunt L, Webb L. Synchronous presentation of breast carcinoma and lymphoma in the axillary nodes. Breast. 2006; 15:246–252.

4. Barranger E, Marpeau O, Uzan S, Antoine M. Axillary sentinel node involvement by breast cancer coexisting with B-cell follicular lymphoma in nonsentinel nodes. Breast J. 2005; 11:227–228.

5. Cohen PL, Brooks JJ. Lymphomas of the breast: a clinicopathologic and immunohistochemical study of primary and secondary cases. Cancer. 1991; 67:1359–1369.

6. Cheung KJ, Tam W, Chuang E, Osborne MP. Concurrent invasive ductal carcinoma and chronic lymphocytic leukemia manifesting as a collision tumor in breast. Breast J. 2007; 13:413–417.

8. Tamaoki M, Nio Y, Tsuboi K, Nio M, Tamaoki M, Maruyama R. A rare case of non-invasive ductal carcinoma of the breast coexisting with follicular lymphoma: a case report with a review of the literature. Oncol Lett. 2014; 7:1001–1006.

9. Arana S, Vasquez-Del-Aguila J, Espinosa M, Peg V, Rubio IT. Lymphatic mapping could not be impaired in the presence of breast carcinoma and coexisting small lymphocytic lymphoma. Am J Case Rep. 2013; 14:322–325.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download