Abstract
Purpose
To investigate treatment options for local control of metastasis in the brain, we compared focal brain treatment (FBT) with or without whole brain radiotherapy (WBRT) vs. WBRT alone, for breast cancer patients with tumor relapse in the brain. We also evaluated treatment outcomes according to the subtypes.
Methods
We conducted a retrospective review of breast cancer patients with brain metastasis after primary surgery. All patients received at least one local treatment for brain metastasis. Surgery or stereotactic radiosurgery was categorized as FBT. Patients were divided into two groups: the FBT group received FBT±WBRT, whereas the non-FBT group received WBRT alone. Subtypes were defined as follows: hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative, HR-positive/HER2-positive, HR-negative/HER2-positive, and triple-negative (TN). We examined the overall survival after brain metastasis (OSBM), brain metastasis-specific survival (BMSS), and brain metastasis-specific progression-free survival (BMPFS).
Results
A total of 116 patients were identified. After a median follow-up of 50.9 months, the median OSBM was 11.5 months (95% confidence interval, 9.0-14.1 months). The FBT group showed significantly superior OSBM and BMSS. However, FBT was not an independent prognostic factor for OSBM and BMSS on multivariate analyses. In contrast, multivariate analyses showed that patients who underwent surgery had improved BMPFS, indicating local control of metastasis in the brain. FBT resulted in better BMPFS in patients with HR-negative/HER2-positive cancer or the TN subtype.
Conclusion
We found that patients who underwent surgery experienced improved local control of brain metastasis, regardless of its extent. Furthermore, FBT showed positive results and could be considered for better local control of brain metastasis in patients with aggressive subtypes such as HER2-positive and TN.
Breast cancer is the most common cancer in women worldwide, and survival is strongly determined by the presence of distant metastases [1,2]. In the United States, as of January 2012, 41% of female cancer survivors had a history of breast cancer. According to 2010 Korean statistics, breast cancer accounts for 14,208 (14.3%) new cases and 102,946 (19.6%) prevalent cases in women [3].
Breast cancer represents the second most frequent cause of brain metastasis (BM) [4]. Among distant metastases, BM is less common than bone or visceral metastasis, but has poor prognosis and survival rates. Historically, survival of patients diagnosed with BM has been quite poor, and the median survival after BM from breast cancer in non-treated patients is reported to be 1 month [5]. The incidence of symptomatic BM is estimated to be 10% to 16%, while autopsy diagnosis shows an incidence of up to 30% [6,7]. With improved systemic therapies and neuroimaging, BM incidence is increasing in breast cancer patients. From 2005 to 2010, BM rates have increased to 25% to 34% [2,8], and BM typically occurs in the late stages of metastatic breast cancer. In most cases, BM is followed by metastases to other organs such as the liver, lungs, or bone. As systemic therapies for control of extracranial disease improve, an increasing number of human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients experience prolonged survival and up to half develop BM over time [6,7,8,9,10].
Therefore, there is an increasing need to standardize initial treatments for breast cancer BM. General treatment guidelines may be divided by prognosis of patients and extent of brain metastatic disease; however, these guidelines are not specific to breast cancer [11]. For limited BM (≤4), surgical resection or stereotactic radiosurgery (SRS) is used as the primary treatment, and both these methods are collectively referred to as focal brain treatment (FBT). For patients with extensive BM, such as multiple BM or leptomeningeal metastases, palliative whole brain radiotherapy (WBRT) and palliative care are recommended. Systemic therapy options are considered for patients with good performance status [12]. Other prognostic factors associated with poor survival after BM include poor performance status and triple-negative (TN) subtype [13].
Consequently, more information on treatment outcome is needed to optimize local therapeutic strategies for patients with breast cancer BM. In this study, we report the results of a retrospective study comparing FBT±WBRT vs. WBRT alone, for breast cancer patients with tumor relapse in the brain. Furthermore, to explore the influence of tumor biology on local treatment outcome, we also evaluated the effect of different cancer subtypes.
We retrospectively reviewed the medical records of breast cancer patients diagnosed with BM at Severance Hospital and Gangnam Severance Hospital, Yonsei University Medical College, Seoul, Korea, between January 2005 and December 2012. During this period, 127 patients were diagnosed with BM, irrespective of other solid organ metastasis. Of these, 11 patients (8.7%) were treated with systemic chemotherapy only, leading to exclusion; therefore, 116 women were eligible for analyses. All reviewed patients underwent surgery for primary breast cancer. Patients were excluded if they had distant metastases at the time of initial diagnosis, incomplete pathologic results, or no treatment after diagnosis of BM. Additionally, patients presenting with leptomeningeal seeding at initial BM diagnosis were excluded.
All patients had histologically confirmed adenocarcinoma of the breast. Clinical data gathered included patients' characteristics, diagnosis date, and initial tumor staging according to the TNM staging system (American Joint Committee on Cancer, 7th edition, 2010). Tumor characteristics including the histologic grade and immunohistochemical staining for estrogen receptor (ER), progesterone receptor (PR), and HER2 were investigated, along with local treatment methods for BM. The histologic grade was evaluated by using the Nottingham combined histologic grading system, which determines the grade by assessing morphologic features (tubule formation, nuclear polymorphism, and mitotic count) and classifies tumors as grades I-III. The ER and PR positivity status was defined by an Allred score of 3-8. The HER2 status was evaluated using antibody staining and/or fluorescence in situ hybridization. The TN subtype was defined as a lack of ER, PR, and HER2 expression. These biomarkers were evaluated using tissues from the primary tumor. Tumors were classified into four subtypes: hormone receptor (HR)-positive/HER2-negative, HR-positive/HER2-positive, HR-negative/HER2-positive, and TN. The Institutional Review Board (IRB) of Severance Hospital, Korea, approved the study in accordance with good clinical practice guidelines and the Declaration of Helsinki (local IRB number: 3-2014-0575).
Routine screening for BM was not performed. When clinically suspected, brain magnetic resonance imaging and/or computerized tomography were performed to confirm the diagnosis of BM. The most common symptom associated with BM was headache, and other reported symptoms included mental status change, cognitive disturbance, and visual disturbance. We counted the number of metastatic lesions in the brain based on imaging taken at the initial BM diagnosis.
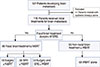
All BM patients received at least one local treatment, which consisted of surgery, SRS, or WBRT. They were classified into two types: surgery or SRS was categorized as FBT, and treatment with WBRT alone was categorized as non-FBT. Depending on the type of local treatment administered, patients were divided into two groups: FBT and non-FBT. The FBT group received FBT with or without WBRT; this group was divided further into three subgroups: surgery, SRS, and surgery and SRS. The consort diagram summarizes these classifications (Figure 1).
Patients' characteristics were compared using the log-rank test and Cox proportional hazards regression model. Overall survival after BM (OSBM) was defined as the time from initial BM diagnosis to the time of death or last follow-up. BM-specific survival (BMSS) was defined as the time from the initial BM diagnosis to the time of death resulting from BM. This was assessed by reviewing charts where the main cause of death was identified. Cases with evidence of BM progression prior to death were regarded as BM-related deaths. BM-specific progression-free survival (BMPFS) was defined as the time from the initial BM diagnosis to the time of BM progression. This was confirmed using magnetic resonance imaging and/or computed tomography. Univariate survival analysis was performed to identify prognostic factors using the log-rank test. Multivariate survival analysis was performed to assess the effects of breast cancer subtypes and other prognostic factors influencing survival using a Cox proportional hazards regression model. The hazard ratios for prognostic factors and the corresponding 95% confidence intervals (CI) were estimated. All analyses were performed using the SPSS version 20.0 (IBM Corp., Armonk, USA) program and p-values less than 0.05 were considered statistically significant.
From January 2005 to December 2012, a total of 116 breast cancer patients newly diagnosed with BM, who had received local treatment for BM were identified. The patients' characteristics are summarized in Table 1. The median age at diagnosis of initial breast cancer was 45 years (range, 25-69 years). The median follow-up period from initial breast cancer diagnosis was 50.9 months (range, 7.1-240.6 months). The median age at BM diagnosis was 46 years (range, 28-71 years), and the median time to BM after initial diagnosis was 35.4 months (range, 6.0-225.7 months). In the study population, 57 (49.1%) and 47 (40.5%) were ER- and HER2-positive, respectively, and 40 (34.5%) had TN cancer.
The brain was the first metastatic site in 19 patients (16.3%), and in 97 (83.7%), BM occurred after systemic metastases. The major sites of systemic disease preceding BM were the lungs (62.1%), bone (54.3%), and liver (24.7%). At the time of BM diagnosis, the 19 patients (16.3%) did not have any other combined metastases. Further, 51 patients (43.9%) had only one additional metastasis at initial BM diagnosis. Of these, the lungs were the most common metastatic site (20 patients, 39.2%). Bone metastasis was observed in 15 patients (29.4%), and lymph node or soft tissue metastasis in 13 patients (25.5%). Liver metastasis was noted in three patients (5.9%). The remaining 46 patients (39.7%) presented with multiple metastases at the initial BM diagnosis.
Local treatments were administered according to the extent of BM and patients with a single metastasis received FBT. Of 45 patients with multiple BM, 32 patients (71.1%) received WBRT alone (Table 2).
Of the 116 total patients examined, 66 (56.9%) received FBT±WBRT for BM, while 50 (43.1%) received WBRT alone. In the FBT group, 18 women were treated with surgery, 38 with SRS, and 10 with surgery and SRS (Figure 1).
At the time of analysis, 99 of the 116 patients were deceased. Causes of death were reviewed, and we found that most patients had died from complications due to metastatic disease. BM was determined to be responsible for the death of 51 patients (51.5%). Deaths due to BM included patients with evidence of BM progression prior to death. Lung and liver metastasis caused death in 27 (27.3%) and 15 patients (15.2%), respectively. The remaining six patients (6.0%) died of underlying medical conditions that were not related to metastatic disease.
The median OSBM was 11.5 months (95% CI, 9.0-14.1 months). The Kaplan-Meier OSBM rates at 1 and 2 years were 48.8% (95% CI, 44.1-53.5) and 19.7% (95% CI, 15.8-23.6), respectively.
The median OSBM for the FBT and non-FBT groups was 16.8 months (95% CI, 12.0-21.6 months) and 5.7 months (95% CI, 0.5-11.0 months), respectively (p<0.001, log-rank test) (Figure 2A). Furthermore, for patients in the FBT group, the median OSBM was the highest for women treated with surgery (p=0.003) (Figure 2B). ER, PR, BM number, and subtype were also identified as significant prognostic factors on univariate analyses (Table 1). Multivariate analysis did not show better OSBM for patients treated with FBT; however, positive PR and single brain lesions were associated with a better outcome (Supplementary Table 1).
The median BMSS was 22.6 months (95% CI, 18.4-26.7 months). The median BMSS for the FBT and non-FBT groups was 26.8 months (95% CI, 19.9-33.8 months) and 18.4 months (95% CI, 12.6-24.3 months), respectively (p=0.006, the log-rank test). On univariate analyses for BMSS, histologic grade, PR, and BM number were identified as significant prognostic factors (Table 1). On multivariate analysis, FBT did not significantly prolong BMSS; however, the number of metastases and PR status were significant factors for BMSS (Supplementary Table 1).
The median BMPFS was 10.0 months (95% CI, 7.5-12.5 months). The Kaplan-Meier BMPFS at 1 year was 43.5% (95% CI, 38.4-48.6). To identify treatment outcome according to the type of local treatment administered to control BM, we analyzed the BMPFS according to FBT. On univariate analyses, BMPFS did not correlate significantly with the FBT status (Figure 2C). However, BMPFS significantly differed according to four types of local treatment (Figure 2D). On multivariate analysis, patients who received surgery showed significantly improved BMPFS (hazard ratio, 0.31; 95% CI, 0.11-0.82) (Table 3). This analysis revealed that the PR status and the extent of BM were also significant factors for BMPFS (Table 3).
As shown in Table 1, OSBM and BMPFS differed significantly with subtype. Patients with HR-positive/HER2-negative disease exhibited the best OSBM and BMPFS (Supplementary Figure 1). In contrast, patients with TN disease had the worst outcome. On univariate analysis, BMSS did not significantly differ by subtype.
To examine the prognostic effect of subtype, we compared treatment outcome according to FBT for each subtype. OSBM differed significantly with FBT in patients with HR-positive/HER2-negative (p=0.035) and TN (p=0.028) subtypes (Supplementary Figure 2). With respect to BMSS, the FBT group showed a better outcome in the TN subtype only (p=0.004). Finally, BMPFS differed significantly with FBT in patients with the HR-negative/HER2-positive (p=0.025) or TN (p=0.001) subtypes (Figure 3).
In this study, we found that survival after BM was better in patients who had received FBT than WBRT alone. In addition, we evaluated BMSS and BMPFS to minimize selection bias associated with our retrospective design. BMSS, which was associated with BM control, was better in patients treated with FBT. In addition, BMPFS, which directly reflects BM control, was prolonged in patients who underwent surgery, independent of the BM and PR status.
Furthermore, we evaluated treatment outcome according to the subtypes. In concordance with previous reports, patients with TN disease exhibited the worst OSBM and BMPFS, and surprisingly, FBT prolonged both BMSS and BMPFS. These findings were translated into an enhanced OSBM, despite the small number of patients (n=40). FBT also prolonged BMPFS, but not OSBM, in HR-negative/HER2-positive patients. It is assumed that there were too few patients with this subtype (n=16) to detect a significant change.
On multivariate analyses for OSBM and BMSS, the FBT patients did not show a better outcome than that shown by non-FBT patients, whereas FBT was a significant prognostic factor for both on univariate analyses. This might be associated with patient selection bias because the treatment modalities employed for BM largely depended on its extent; the limited number of patients in the study might also have influenced this result.
Nevertheless, our findings support the general guidelines for BM management, which recommend FBT for limited metastatic tumors in the brain. There is a paucity of data to suggest that these treatments may improve survival in clinical practice. Our study highlights that surgery could improve outcomes for local control of BM, regardless of the number of lesions present. These results support current guidelines and contribute to optimization of treatment modalities for BM in breast cancer patients.
Previous studies have reported several risk factors associated with the development of BM, such as young age (<40 years), high tumor grade, ER negativity, TN subtype, and HER2 overexpression [6,13,14,15]. Our results show a higher prevalence of ER negativity and HER2 overexpression in the general breast cancer population. Among the study population, ER positivity was seen in 57 patients (49.1%). Approximately 65% of all breast cancer patients are thought to be ER-positive; our study showed a lower rate of ER positivity in BM patients. Our data also showed a higher prevalence of HER2 overexpression (47 patients, 40.5%) and TN breast cancer (40 patients, 34.5%) than the general breast cancer population (20%-30% and 20%, respectively) [6]. Considering prognostic factors, our study showed that the TN subtype was a significant factor on univariate analyses for OSBM, BMSS, and BMPFS, but not on multivariate analyses. Moreover, we found that a negative PR status was a poor prognostic factor for survival after BM in breast cancer. The prognostic significance of PR, a well-known biomarker for endocrine responsiveness, and an important prognostic marker for ER-positive breast cancer, appears to be sustained even after BM development.
Limitations of this study include bias inherent to the retrospective nature of the design. The number of patients in the study was limited to those diagnosed with breast cancer BM at two institutions between 2005 and 2012. Bias in the study population could have confounded the results. Neurocognitive assessments were not available and this could have affected treatment planning, thereby creating a selection bias. The cause of death was open to subjective interpretation from reviewing the charts. No autopsies were performed to identify the true nature of death.
Nevertheless, our study provides unique evidence that different types of local treatment can affect the outcome for control of BM. Furthermore, FBT could be considered for better local control of BM in aggressive subtypes such as the HER2 and TN. Our data contributes to the optimization of treatment modalities for BM in breast cancer.
Figures and Tables
 | Figure 2Kaplan-Meier plots showing overall survival after brain metastasis and brain metastasis-specific progression-free survival for local treatment. All p-values were obtained by the log-rank test. (A) FBT vs. non-FBT, p<0.001. (B) Surgery vs. SRS vs. Surgery and SRS vs. WBRT alone, p=0.003. (C) FBT vs. non-FBT, p=0.152. (D) Surgery vs. SRS vs. Surgery and SRS vs. WBRT alone, p=0.001.FBT=focal brain treatment; SRS=stereotactic radiosurgery; WBRT=whole brain radiotherapy.
|
 | Figure 3Kaplan-Meier plots showing brain metastasis-specific progression-free survival for local treatment, subdivided by subtype. All p-values were obtained by the log-rank test. (A) HR+HER2-, p=0.143. (B) HR+HER2+, p=0.515. (C) HR-HER2+, p=0.025. (D) Triple-negative, p=0.001.HR=hormone receptor; HER2=human epidermal growth factor receptor 2; FBT=focal brain treatment; TNBC=triple-negative breast cancer.
|
Table 1
Clinicopathological characteristics

Table 2
Types of local treatment according to the extent of brain metastasis

| Treatment | Extent of brain metastasis | p-value | ||
|---|---|---|---|---|
| Single (n = 27) | Oligometastasis* (n = 44) | Multiple† (n = 45) | ||
| <0.001 | ||||
| Surgery | 7 | 7 | 4 | |
| SRS | 7 | 22 | 8 | |
| SRS+Surgery | 7 | 3 | 1 | |
| WBRT alone | 6 | 12 | 32 | |
Table 3
Multivariate analyses for brain metastasis-specific progression-free survival

References
1. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012; 62:220–241.

2. Xue J, Peng G, Yang JS, Ding Q, Cheng J. Predictive factors of brain metastasis in patients with breast cancer. Med Oncol. 2013; 30:337.

3. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.

4. Hofer S, Pestalozzi BC. Treatment of breast cancer brain metastases. Eur J Pharmacol. 2013; 717:84–87.

5. Karam I, Hamilton S, Nichol A, Woods R, Speers C, Kennecke H, et al. Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the Pre-Trastuzumab and Trastuzumab eras. Radiat Oncol. 2013; 8:12.

6. Ahn HK, Park YH, Lee SJ, Park S, Maeng CH, Park W, et al. Clinical implication of Time To Brain Metastasis (TTBM) according to breast cancer subtypes. Springerplus. 2013; 2:136.

7. Padovani L, Muracciole X, Régis J. , knife radiosurgery of brain metastasis from breast cancer. Prog Neurol Surg. 2012; 25:156–162.
9. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500.

10. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993; 33:583–590.

11. Central nervous system cancers, version 1. National Comprehensive Cancer Network;2013. Accessed August 21st, 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
12. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012; 82:2111–2117.

13. Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013; 112:467–472.

Supplementary Material
Supplementary Table 1
Multivariate analyses for overall survival after brain metastasis and brain metastasis-specific survival after brain metastasis
Supplementary Figure 1
Kaplan-Meier plots showing overall survival after brain metastasis (OSBM) and brain metastasis-specific progression-free survival (BMPFS), subdivided by subtypes. All p-values were obtained by the log-rank test. (A) OSBM, p=0.002. (B) BMPFS, p<0.001.
HR=hormone receptor; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer.
Supplementary Figure 2
Kaplan-Meier plots showing overall survival after brain metastasis (OSBM) for types of local treatment, subdivided by subtype. All p-values were obtained by the log-rank test. (A) HR+HER2-, p=0.036. (B) HR+HER2+, p=0.319. (C) HR-HER2+, p=0.391. (D) Triple-negative, p=0.028.
HR=hormone receptor; HER2=human epidermal growth factor receptor 2; FBT=focal brain treatment; TNBC=triple-negative breast cancer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download