Abstract
Purpose
This study aimed to evaluate the survival benefit of different adjuvant chemotherapy regimens in patients with T1-2N0 triple-negative breast cancer.
Methods
Of 67,321 patients who were registered in the Korean Breast Cancer Society nationwide database between January 1999 and December 2008, 4,033 patients with T1-2N0 triple-negative breast cancer were included. The overall survival of patients who did not receive adjuvant chemotherapy was compared with those treated with adjuvant anthracycline and cyclophosphamide (AC), 5-fluorouracil, anthracycline, and cyclophosphamide (FAC), or cyclophosphamide, methotrexate, and 5-fluorouracil (CMF).
Results
The median follow-up was 52.5 months. Chemotherapy was used in 87.4% of patients; it was used more commonly in patients with T2 tumors, those who were younger, had a higher histologic grade, and who showed lymphovascular invasion. The 5-year cumulative overall survival rate was 95.4%. Younger age, breast-conserving surgery, and adjuvant chemotherapy were significantly associated with improved overall survival. The 5-year cumulative overall survival rate of patients who did not receive adjuvant chemotherapy and those treated with AC, FAC, and CMF were 92.5%, 95.9%, 95.3%, and 95.9%, respectively. On multivariate analysis, the administration of any adjuvant chemotherapy regimen was significantly associated with improved overall survival (p=0.038). No significant difference in survival benefit was observed among the three different treatment groups.
Breast cancer is a heterogeneous disease. Molecular subtyping based on the hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) statuses is very important to determine a treatment strategy for breast cancer. Molecular-targeted therapy has improved patient survival in the adjuvant setting. For patients with HR-positive breast cancer, use of tamoxifen for 5 years can reduce the annual recurrence rate by approximately half and the breast cancer mortality by a third [1]. Aromatase inhibitors, which are commonly used for postmenopausal patients with HR-positive disease, improve the disease-free survival in this population [2]. In combination with chemotherapy, adjuvant HER2-targeted therapy with either concomitant or sequential trastuzumab significantly improves the disease-free survival and overall survival of patients with HER2-positive breast cancer [345]. However, triple-negative breast cancer (TNBC) remains a challenging problem for both clinicians and investigators. TNBC, characterized by the absence of the estrogen receptor (ER), the progesterone receptor (PR), and HER2, represents approximately 10% to 20% of breast cancer cases [6]. It is associated with poor survival even in patients with stage I breast cancer [7]. Owing to the absence of targeted therapeutic agents, conventional chemotherapy remains the main treatment for TNBC.
Adjuvant chemotherapy is a well-established and effective treatment for the management of breast cancer, even in ER-positive cases [8]. Nevertheless, the toxicity and high cost of chemotherapy are potential obstacles of use, for both physicians and patients. To avoid overtreatment with chemotherapy, studies to identify patients who may benefit from adjuvant chemotherapy are currently underway. Although there are promising results with gene expression profiling, these studies have not yet been shown to help select patients with node-negative TNBC who could be good candidates for adjuvant chemotherapy [9]. In addition, there is no universally accepted standard adjuvant chemotherapy regimen for the treatment of TNBC because of a lack of reliable evidence in support of a particular regimen. The use of platinum-based chemotherapy, epidermal growth factor receptor targeted-agents, and other novel agents in the adjuvant treatment of TNBC are still under investigation [10]. Standard adjuvant chemotherapy regimens such as the classic cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), anthracycline-containing, and taxane-containing regimens are currently accepted as reasonable choices in routine practice for patients with TNBC [10]. Nonetheless, controversy still exists about choosing an adjuvant chemotherapy regimen with an acceptable level of adverse toxicity for a low-risk population such as patients with relatively small node-negative tumors.
As there are currently no available evidence-based preferred regimens for TNBC, selection of chemotherapy is commonly based on pathologic parameters such as the nodal status and tumor size. According to most clinical guidelines, all patients with node-positive disease and/or tumors >1 cm should be considered for adjuvant chemotherapy regardless of the molecular subtype [11]. Despite this recommendation, there is no universally accepted protocol to aid in the selection of adjuvant chemotherapy regimens for patients with node-negative TNBC.
The purpose of our study was to evaluate the influence of different systemic adjuvant chemotherapy regimens on the survival of patients with T1-2 node-negative TNBC enrolled from the Korean Breast Cancer Society (KBCS) nationwide database.
Patients with newly diagnosed invasive ductal or lobular, T1-2 node-negative TNBC between January 1999 and December 2008 were identified from the KBCS database. The KBCS database has prospectively collected nationwide breast cancer data since 1996. It includes information about patient sex; age at diagnosis; cancer stage according to the sixth American Joint Committee on Cancer classification; type of surgical procedure; histologic findings; ER, PR, HER2, and other biological marker status; adjuvant treatment; and other miscellaneous data. Patient survival data were provided by the Death Certification of the Korean National Statistical Office and the Korean Central Cancer Registry of the Ministry of Health and Welfare [12]. One hundred fourteen institutes participate in this KBCS database project. Detailed information on the KBCS database has been provided previously [1213]. ER and PR status was defined as positive or negative according to the standard method of each institution. HER2 positivity was defined as a 3+ score on immunohistochemical analysis and/or gene amplification observed with fluorescence in situ hybridization. Each physician prospectively registered information regarding the ER, PR, and HER2 status in the KBCS database, according to institutional standards. This retrospective study was approved by the Institutional Review Board of the Korea Cancer Center hospital (K-1305-002-017).
Between January 1999 and December 2008, 51,739 patients with newly diagnosed invasive ductal or lobular breast cancers were registered in the KBCS database. We excluded patients with incomplete information regarding the ER, PR, and HER2 statuses; lymph node metastasis; and adjuvant chemotherapy regimens. Male patients and patients with other previous malignancies, lymph node metastasis, or who underwent treatment with adjuvant chemotherapy regimens other than CMF, anthracycline and cyclophosphamide (AC), or 5-fluorouracil, anthracycline, and cyclophosphamide (FAC) were also excluded. A score of 0-1+ on immunohistochemical analysis or a negative result after fluorescent in situ hybridization confirmed the TNBC ER-, PR-, and HER2-negative statuses. Of these patients, 4,033 patients with TNBC were included in this analysis (Figure 1). The last date of follow-up for survival was December 31, 2009. The primary endpoint of this study was death caused by any reason. As the KBCS database only provides patients' survival information, analysis of disease recurrence could not be performed.
Data analysis was performed with SPSS version 14.0 (SPSS Inc., Chicago, USA). The chi-square test was used to assess differences in values between the groups. Survival rates were estimated by using the Kaplan-Meier method, and compared with the log-rank test. Multivariate analyses were performed with the linear logistic regression model and the Cox proportional hazards model. Statistical significance was accepted as p-values of <0.05.
Among 51,739 patients with newly diagnosed invasive ductal or lobular breast cancer in the KBCS database, the data from 18,867 patients were available for this analysis. Of these patients, 21.4% (4,033/18,867) were classified as having T1-2N0 TNBC (Figure 1). The mean age at diagnosis was 48.5±10.8 years (range, 21-91 years). Most of the tumors were T1 (53.6%) and had a high histological grade (grade 3; 57.0%). Lymphovascular invasion was observed in 591 patients (14.7%). Breast-conserving surgery (BCS) was performed in 57.8% of the patients (Table 1).
Overall, 3,781 patients (86.4%) were treated with adjuvant chemotherapy. AC was the most commonly administered chemotherapy regimen with 1,419 patients (35.2%) receiving this treatment. CMF was administered to 1,190 patients (29.5%). FAC was used as an adjuvant treatment for 875 patients (21.7%) (Table 1). Chemotherapy was administered to 91.3% (879/963), 88.9% (1,310/1,473), 87.5% (911/1,029), 78.3% (346/442), and 38.0% (46/121) of patients aged ≤40, 41-50, 51-60, 61-70, and >71 years, respectively. Adjuvant chemotherapy was more frequently used in patients aged ≤50 years, and in patients who had larger tumors (T2), high histologic grade tumors, tumors showing lymphovascular invasion, and who were treated with BCS (Table 2). In a multivariate model, values significantly predictive of adjuvant chemotherapy use included younger age, T2 tumor, high histologic grade, lymphovascular invasion, and BCS (Table 2).
The AC and FAC regimens were more commonly used for patients with high-risk characteristics such as younger age, larger tumor size, higher histologic grade tumors, and the presence of lymphovascular invasion. In contrast, the CMF regimen was more frequently used in patients aged >50 years, and in patients with smaller tumors (T1), lower histologic grade tumors, and no lymphovascular invasion (Table 3).
After a median follow-up of 52.5 months, there were 150 deaths, including 11 unrelated deaths, 69 breast cancer-related deaths, and 70 deaths due to unknown reasons. Of the 11 unrelated deaths, heart problems were the cause of death in only one patient; the patient was 65 years at diagnosis, was not treated with adjuvant chemotherapy, and died 6 years after the operation. The 5-year overall survival rate was 95.4% (Figure 2). Age >50 years, mastectomy, and no adjuvant chemotherapy were associated with a significantly increased risk of death on univariate analysis (Table 4). Compared to the T1 stage, T2 showed a greater risk of death but this result did not reach statistical significance (p=0.058).
Compared to no adjuvant chemotherapy, adjuvant chemotherapy with any regimen had a beneficial effect on overall survival (Table 4). The beneficial effect of adjuvant chemotherapy was retained after adjustment for age, T stage, and surgical procedures (Table 5). When compared with patients who did not receive adjuvant chemotherapy, the hazard ratio of death was 0.524 (95% confidence interval [CI], 0.325-0.846; p=0.008) in the AC group, 0.516 (95% CI, 0.292-0.912; p=0.023) in the FAC group, and 0.567 (95% CI, 0.357-0.900, p=0.016) in the CMF group.
There was no significant difference in patient survival rates between the chemotherapy regimens employed (AC vs. FAC, p=0.907; AC vs. CMF, p=0.589; FAC vs. CMF, p=0.654) (Table 6, Figure 3). The multivariate analysis of overall survival in patients treated with adjuvant chemotherapy showed no difference in the survival benefit between the adjuvant chemotherapy regimens (Table 7).
In our study, adjuvant chemotherapy was administered to 86.4% of patients with T1-2N0 TNBC in South Korea, and it significantly improved the overall survival in this retrospective cohort. No difference in patient survival was observed between the various adjuvant chemotherapy regimens used. An advantage of this study is that it only included patients with node-negative TNBC from a large nationwide cohort. There are only a few studies examining adjuvant chemotherapy for node-negative breast cancer because this group has a better prognosis. In addition, most of these studies have focused on patients with ER-negative breast cancer, resulting in study cohorts with a mixture of ER-/HER2+ and TNBC patients. In this era of HER2-targeted therapy, the inclusion of a HER2 overexpression group in this analysis could cause confusion about the effects of chemotherapy on survival.
According to our analysis, the use of adjuvant chemotherapy was associated with an improved overall survival rate, which is in agreement with a previous large retrospective analysis [14]. The International Breast Cancer Study Group conducted a retrospective study to evaluate the efficacy of CMF for each molecular subtype in patients with node negative disease enrolled in two randomized clinical trials [14]. A significant reduction in the hazard ratio for chemotherapy versus no chemotherapy was observed for the TNBC subtype (hazard ratio, 0.46; 95% CI, 0.29-0.73) [14].
The efficacy of CMF, AC, and FAC was similar in our study. This result corresponds with the findings of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-23, which was designed to determine whether AC was comparable or superior to CMF when used to treat patients with node-negative ER-negative breast cancer. They demonstrated no statistically significant differences in the outcomes of AC-treated patients and CMF-treated patients during 8 years of follow-up [1516]. A retrospective study analyzed the responsiveness of different breast cancer subtypes to specific chemotherapy regimens in the MA5 adjuvant trial [17]. There were no significant differences in the disease-free survival (hazard ratio, 1.1; 95% CI, 0.6-2.1) and overall survival (hazard ratio, 1.3; 95% CI, 0.7-2.5) between cyclophosphamide-epirubicin-5-fluorouracil and CMF in basal-like tumors. Together with the findings of our study, these results suggest that any of these regimens (CMF, AC, or FAC) is a reasonable therapeutic choice in terms of efficacy.
When there is no difference in the efficacy between different treatments, drug-related toxicities should be considered when selecting the optimal treatment regimen. The classic CMF regimen has been a standard treatment for a long time, and the safety of this regimen is well known [1819]. Comparisons of drug compliance and toxicity between doxorubicin-cyclophosphamide and CMF were performed in the NSABP B-23 study. The toxicity of four courses of AC therapy was similar to that of classic CMF [16]. In contrast, FAC treatment significantly induced more episodes of emesis, mucositis, alopecia, and cardiotoxicity compared with CMF [2021]. Extending the AC chemotherapy regimen from four to six cycles increased toxicity, including cardiotoxicity, without survival improvement [22]. As the results of our study showed that the efficacy of FAC was not different from either that of AC or CMF, these latter treatments with less adverse toxicity may be considered a better choice for the low-risk group of patients in our study, and particularly for older patients.
Cardiotoxicity is an important issue when studies of adjuvant anthracycline-containing chemotherapy are conducted. Anthracycline-induced cardiotoxicity could not be assessed in our study because 46.7% of deaths were caused by unknown reasons. Only one death was known to result from a heart-related problem.
Although many drugs such as platinum-based drugs, taxanes, bevacizumab, epidermal growth factor receptor-targeted agents, and poly adenosine diphosphate ribose polymerase inhibitors are being investigated as potential therapeutic agents for TNBC, there is no generally accepted adjuvant regimen for TNBC [10]. Most clinical trials to test these novel agents are conducted in high-risk patients; therefore, it would take a long time to consider the results and adapt these drugs to adjuvant regimens that could be used in routine clinical practice for low-risk patients. Until then, physicians and patients have to choose one of the conventional chemotherapies.
This study has some limitations. Firstly, regimens using taxanes were excluded from the analysis. In Korea, the use of taxanes in the adjuvant setting for node-negative breast cancer is not supported by the National Health Insurance Service. Only 78 patients with T1-2 node-negative TNBC received adjuvant treatment with AC followed by taxane or anthracycline-taxane. There are limited data on the adjuvant use of taxanes in patients with node-negative breast cancer, which remains controversial [22232425]. Therefore, we excluded these patients as well as a small number treated with different regimens containing agents less frequently used in this population, such as oral 5-fluorouracil, vinorelbine, capecitabine, and gemcitabine. Secondly, TNBC was classified by data that was collected at each institute since 1999. The KBCS database recommends the results of ER, PR, or HER2 examinations should be classified according to each institute's guideline and has no specific pathological validation program. Therefore, we assume that part of data would not match with the American Society of Clinical Oncology/College of American Pathologists guideline for HER2, which was written in 2007. In addition, the exact dose, cycles, and routes of administration of adjuvant chemotherapy were not provided by the KBCS database. We analyzed the survival data based on only the chemotherapy regimen. Lastly, patients with T1a/b tumors were included in the analysis, and the merit of adjuvant chemotherapy in these patients is debatable. Administration of adjuvant chemotherapy was not associated with a significantly higher recurrence rate in T1a/bN0 HER2-positive breast cancer or TNBC [262728]. We incorporated this group of patients because the KBCS database has only limited data on T stage subclassifications, even though the inclusion of this population might dilute the results of the analysis.
In conclusion, currently used standard adjuvant chemotherapy regimens improved the overall survival in patients with T1/2 node-negative TNBC. There was no significant difference in the survival of patients administered CMF, AC, or FAC regimens. In the future, randomized prospective studies should lead to the development of new adjuvant treatment modalities for low-risk patients with TNBC. Currently, the selection of standard chemotherapy regimens with fewer drug-related adverse toxicities may be the most reasonable choice.
Figures and Tables
 | Figure 1Study design.IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; CMF=cyclophosphamide, methotrexate, and 5-fluorouracil; FAC=5-fluorouracil, anthracycline, and cyclophosphamide; AC=anthracycline and cyclophosphamide.
|
 | Figure 3Overall survival according to the chemotherapy regimen used. The receiving adjuvant chemotherapy showed beneficial effect on overall survival compared with not receiving adjuvant chemotherapy. There was no significant difference in the survival rates between the chemotherapy regimens employed.CMF=cyclophosphamide, methotrexate, and 5-fluorouracil; FAC=5-fluorouracil, anthracycline, and cyclophosphamide; AC=anthracycline and cyclophosphamide.
|
Table 1
Patients characteristics

Table 2
Factors associated with use of adjuvant chemotherapy

Table 3
Clinicopathologic characteristics according to adjuvant chemotherapy regimens
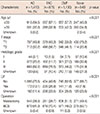
Table 4
Univariate analysis of overall survival in all patients

Table 5
Multivariate analysis of overall survival in all patients

Table 6
Univariate analysis of overall survival in patients treated by adjuvant chemotherapy

References
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717.
2. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11:1135–1141.

3. Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011; 29:3366–3373.

4. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283.

5. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 Years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013; 382:1021–1028.

6. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010; 7:683–692.

7. Kim JE, Ahn HJ, Ahn JH, Yoon DH, Kim SB, Jung KH, et al. Impact of triple-negative breast cancer phenotype on prognosis in patients with stage I breast cancer. J Breast Cancer. 2012; 15:197–202.

8. Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012; 379:432–444.

9. Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013; 5:61–70.

10. Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol. 2012; 23:Suppl 6. vi40–vi45.

11. Hernandez-Aya LF, Gonzalez-Angulo AM. Adjuvant systemic therapies in breast cancer. Surg Clin North Am. 2013; 93:473–491.

12. Kim HA, Kim EK, Kim MS, Yu JH, Lee MR, Lee HK, et al. Association of human epidermal growth factor receptor 2 with radiotherapy resistance in patients with T1N0M0 breast cancer. J Breast Cancer. 2013; 16:266–273.

13. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368.

14. Colleoni M, Cole BF, Viale G, Regan MM, Price KN, Maiorano E, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010; 28:2966–2973.

15. Fisher B, Jeong JH, Anderson S, Wolmark N. Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst. 2004; 96:1823–1831.

16. Fisher B, Anderson S, Tan-Chiu E, Wolmark N, Wickerham DL, Fisher ER, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001; 19:931–942.

17. Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, Chia SK, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012; 18:2402–2412.
18. Goldhirsch A, Coates AS, Colleoni M, Castiglione-Gertsch M, Gelber RD. International Breast Cancer Study Group. Adjuvant chemoendocrine therapy in postmenopausal breast cancer: cyclophosphamide, methotrexate, and fluorouracil dose and schedule may make a difference. J Clin Oncol. 1998; 16:1358–1362.
19. Colleoni M, Price KN, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A. International Breast Cancer Study Group. Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil. Lancet. 1999; 354:130–131.

20. Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, et al. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003; 14:833–842.
21. Hutchins LF, Green SJ, Ravdin PM, Lew D, Martino S, Abeloff M, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005; 23:8313–8321.
22. Shulman LN, Cirrincione CT, Berry DA, Becker HP, Perez EA, O'Regan R, et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol. 2012; 30:4071–4076.

23. Martín M, Ruiz A, Ruiz Borrego M, Barnadas A, González S, Calvo L, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. J Clin Oncol. 2013; 31:2593–2599.
24. Ozdemir N, Aksoy S, Zengin N, Altundag K. Taxanes in the adjuvant treatment of node-negative breast cancer patients. J BUON. 2012; 17:27–32.
25. Lagha A, Chraiet N, Labidi S, Krimi S, Ayadi M, Gligorov J, et al. Impact of taxanes in the adjuvant setting of node-negative breast cancers. Bull Cancer. 2013; 100:465–471.
26. Migdady Y, Sakr BJ, Sikov WM, Olszewski AJ. Adjuvant chemotherapy in T1a/bN0 HER2-positive or triple-negative breast cancers: application and outcomes. Breast. 2013; 22:793–798.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download