Abstract
Purpose
Metformin may be associated with a decreased risk of breast cancer. We performed a meta-analysis to assess the effect of metformin intake on breast cancer risk and mortality.
Methods
We performed a PubMed and EMbase search for all available studies that described the risk of breast cancer and all-cause mortality in relation to the use of metformin among patients with type 2 diabetes mellitus. Pooled relative risks (RRs) were determined using a random effects model to assess the strength of association between metformin and the risk of breast cancer.
Results
Fifteen articles from PubMed satisfied the inclusion criteria, including a total of 838,333 participants. Compared with the control group, metformin use was not related to a reduced incidence of breast cancer (RR, 0.964; 95% confidence interval [CI], 0.761-1.221; p=0.761). However, metformin therapy was associated with decreased all-cause mortality (RR, 0.652; 95% CI, 0.488-0.873; p=0.004). No obvious publication bias was detected (incidence: pBegg=0.755, pEgger=0.008; mortality: pBegg=0.072, pEgger=0.185).
Breast cancer is a serious challenge in women's health. Its incidence has increased in recent years [1]. Because of the crucial role of hyperinsulinemia, both obesity and type 2 diabetes mellitus have been associated with increased breast cancer risk [2]. Metformin is a widely used drug for the treatment of type 2 diabetes mellitus, and it can effectively decrease circulating levels of glucose and insulin mainly by counteracting insulin resistance. It can also improve insulin sensitivity by increasing peripheral glucose utilization without lowering blood glucose levels in nondiabetic patients. Metformin has aroused great attention also as an anticancer factor.
Numerous studies in vitro and in vivo indicated that metformin may inhibit cancer cell growth and reduce the risk of developing different solid tumors [3]. However, numerous epidemiological studies evaluating the association between metformin and breast cancer have produced controversial results [45]. Therefore, we systematically searched the literature to comprehensively assess the effect of metformin on breast cancer risk and mortality.
Our study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [6]. We searched the PubMed and EMbase databases (from inception to February 10, 2015) for randomized and observational studies that discussed the association between metformin and breast cancer in patients with diabetes mellitus, by using the following search terms: "metformin" and "breast cancer." We limited searches to studies in humans published in English-language journals.
We reviewed all relevant papers. The inclusion criteria were the following: (1) studies on patients with diabetes mellitus that reported data on exposure to metformin therapy, in comparison with a control group, and breast cancer incidence or mortality; (2) studies presenting the relative risk (RR) estimates, and the corresponding 95% confidence intervals (CIs), size of baseline samples, or other information that can help to interpret the results; and (3) studies written in English language. The publications were excluded if they met any of the following criteria: (1) therapy with drugs other than metformin; (2) cancers other than breast; (3) absent focus on the association between metformin and breast cancer; (4) basic or animal research; (5) review; and (6) absence of relevant data. Studies that were not published as full reports were excluded.
The data were extracted independently by two reviewers (T.Y. and Y.Y.), by using predefined data extraction forms. A third party (S.L.) was involved when necessary. The collected information included first author, year of publication, study location, ethnicity of subjects, study period, duration of follow-up, mean age of the baseline sample, number of cases, ascertainment of cases, adjustment for covariates, RR and the corresponding 95% CI. We extracted any reported RRs, hazard ratios [HRs], or incidence density ratios of metformin users outcomes compared with the reference group.
Two authors (T.Y. and Y.Y.) independently assessed the quality of each study based on the Newcastle-Ottawa Scale (NOS) [7]. All the differences were resolved by discussion. A third party (S.L.) was involved when necessary. A "star system" was developed under the NOS and was applied in the present analysis to judge each study included. The quality of the studies was evaluated by examining three items: selection of the study groups (4 criteria), between-group comparability (1 criterion), and the ascertainment of either the exposure (for case-control studies) or the outcome (for cohort studies) (3 criteria). A study could be awarded a maximum of one star for each item within the selection and exposure categories, and a maximum of two stars for comparability. The total NOS star count ranged from zero to nine.
The statistical analysis was based on previously reported methods [8]. The association between metformin therapy and breast cancer was measured by RR. The HRs were considered equivalent to RRs. Heterogeneity across studies was assessed using the Cochran's χ2-based Q test and the I-squared test. Heterogeneity was not considered as significant when p>0.05 or I2<50%. If no significant heterogeneity was found, the pooled RR estimate of each study was calculated using the fixed effects model (Mantel-Haenszel). Otherwise, the random effects model (DerSimonian and Laird) was used [9].
The Egger's linear regression test [10] and the Begg's rank correlation test [11] were used to assess potential publication bias. All statistical tests were conducted using the STATA software version 11 (StataCorp LP, College Station, USA). A p-value of 0.05 for any test or model was considered to be statistically significant.
We identified a total of 299 citations from PubMed, of which 231 were excluded in an initial title and abstract screening, mainly because they were duplicate records, reviews, comments, basic or animal studies, or not relevant to our analysis. After reviewing the full texts in detail, 53 studies were excluded because they did not refer to the association between metformin and the outcomes of interest. Finally, 15 articles [121314151617181920212223242526] were included in the analysis (Figure 1). In the EMbase database, most of the publications were conference abstracts. Moreover, there was a substantial overlap between the Embase and PubMed databases. Therefore, we only analyzed the articles found in PubMed.
All the selected 15 articles (including 18 studies) were published between 2010 and 2014. The studies reported in six publications were conducted in the United States of America, five in the United Kingdom, two in Taiwan, and two in Denmark. One publication [13] included two eligible studies, and one [24] included three eligible studies. Overall, we included 10 case-control studies and eight retrospective cohort studies. The enrolled participants were Caucasian in 10 studies, Asian in two, African American in two, mixed-raced in two. One of the studies did not give information on the participants' ethnicity. At the study baseline, a total of 838,333 participants were involved. Some cases were lost during follow-up in two cohort studies. Eleven studies [181920212223242526], with a total of 175,254 participants, referred to the incidence of breast cancer. Seven studies [121314151617] referred to the mortality of breast cancer, with 6,553 patients died out of 28,076 breast cancer patients. The longest follow-up time was 12 years. Most included studies analyzed the data with adjustment for more than one variable, such as age, age at breast cancer diagnosis, sex, race, body mass index, tobacco use, alcohol use, family history of breast cancer, medication use, estrogen receptor, and progesterone receptor. The details are shown in Tables 1 and 2. In the risk of bias assessment using the NOS, all the studies obtained between five and nine stars, indicating a moderate to high quality.
The combined results of the 11 studies showed that, compared with the control groups, metformin therapy did not significantly reduce the incidence of breast cancer (RR, 0.964; 95% CI, 0.761-1.221; p=0.761), using the random effects model (heterogeneity: I2=90.7%) (Figure 2). The results of the stratification analyses by study design found that metformin therapy was not related to a reduced incidence of breast cancer, neither in case-control studies (RR, 0.933; 95% CI, 0.680-1.279; p=0.666; I2=88.5%, random effects model), nor in cohort studies (RR, 1.022; 95% CI, 0.822-1.270; p=0.844; I2=68.2%, random effects model). The results of the stratification analyses by ethnicity found that metformin therapy was not related to a reduction in the incidence breast cancer, neither in Asian participants (RR, 1.017; 95% CI, 0.371-2.784; p=0.974; I2=92.8%, random effects model), nor in Caucasian participants (RR, 0.969; 95% CI, 0.870-1.079; p=0.566; I2=46.9%, fixed effects model).
Seven studies in six included publications [121314151617] focused on the all-cause mortality. The all-cause mortality seemed to be significantly decreased in metformin users (RR, 0.652; 95% CI, 0.488-0.873; p=0.004) (Figure 3), with the random effects model (heterogeneity: I2=78.9%). Results of the stratification analyses by study design found that metformin therapy was not related to a reduced all-cause mortality of breast cancer patients in cohort studies (RR, 0.926; 95% CI, 0.851-1.007; p=0.071; I2=77.2%, random effects model). However, casecontrol studies yielded significant results (RR, 0.663; 95% CI, 0.526-0.836; p=0.001; I2=75.7%, random effects model). The results of the stratification analyses by ethnicity found that metformin therapy was unrelated with a reduced all-cause mortality of breast cancer in African American participants (RR, 0.366; 95% CI, 0.045-2.995; p=0.349; I2=89.3%, random effects model). However, in Caucasian participants, metformin significantly decreases the all-cause mortality of breast cancer patients (RR, 0.677; 95% CI, 0.497-0.922; p=0.013; I2=61.6%, random effects model).
Our study showed that in observational studies there was no significant association between metformin use and risk of breast cancer. However, exposure to metformin was associated with a 40.4% reduction of all-cause mortality.
A potential metformin influence on breast cancer was supported by emerging clinical studies. One cohort study in the United Kingdom, with a median follow-up time of 5.1 years, showed that individuals with diabetes who used metformin had a similar risk of developing cancer compared with those who used sulfonylureas (HR, 0.96; 95% CI, 0.89-1.04) [26]. Another study in Taiwan found that diabetic patients treated with insulin or sulfonylureas had significantly higher risk of all cancers, compared to those treated with metformin (OR, 1.583, 95% CI, 1.389-1.805, p<0.001; and OR, 1.784, 95% CI, 1.406-2.262, p<0.001) after adjusting for sex and age [22]. Sulfonylureas treatment was associated with an increased risk of breast cancer (OR, 1.784; 95% CI, 1.406-2.262; p<0.001). Xiao et al. [27] analyzed mortality in luminal-type breast cancer patients. The results showed that in the luminal A-subtype group seven patients died in the metformin group (8.3%), versus 26 in the nonmetformin group (22.2%). In the luminal B (high Ki-67)-subtype group, 15 patients died in the metformin group (10.7%) and 65 in the nonmetformin group (32.3%). In the luminal B (HER2/neu+)-subtype group, 11 patients died in the metformin group (21.6%) and 36 in the nonmetformin group (41.4%). The Kaplan-Meier univariate analysis showed a significant difference between metformin and nonmetformin treatment in all three groups (p=0.002). The median follow-up time in this study was 70 months (range, 10-120 months). Another study found significant decrease in mortality risk (HR, 0.762; 95% CI, 0.6-0.968; p=0.026) in metformin group [28]. This study also showed higher mortality risk in patients younger than 35 years old than in patients older than 65 years old (<35 vs. >65; HR, 1.274; 95% CI, 1.049-1.547; p=0.015). In general, these studies suggested that metformin may influence breast cancer.
Unlike most of the previous meta-analyses, our results showed no significant association between metformin use and the risk of breast cancer. It should be noted that we took more outcomes into account, and identified a much larger number of studies and patients. We analyzed case-control studies and cohort studies separately. Furthermore, we considered different ethnicities included in the studies on the association between metformin and breast cancer.
Our findings had substantial clinical and public significance. However, these results should be taken with caution because of the limitations of this meta-analysis and of the included studies. First, we did not evaluate the relationship between different doses of metformin with breast cancer. As glucose-lowering therapies were modified according to glycemic control or side effects, the metformin dose at baseline does not necessarily represent the levels of ongoing exposure during the follow-up period. Second, metformin users were at different stages of diabetes. As compared to patients treated with metformin, patients treated with other antidiabetic drugs were more likely to have severe diabetes or longer disease duration [29], even if most of these studies reported the results of analyses adjusted for the abovementioned confounding factors. The heterogeneity of the comparator populations was also a very important factor affecting the results. The comparator group was defined as "no metformin users" which included insulin, thiazolidinediones, and other antidiabetic drugs. Insulin and thiazolidinediones have been associated with hyperinsulinemia and increased risk of cancer. Finally, the association of different type and characteristics of breast cancers with metformin use was not described in those studies.
Currently, a number of clinical trials examining the potential influence of metformin on breast cancer are underway. The results of the clinical trials will contribute to assess whether metformin can be used as an anticancer agent.
Figures and Tables
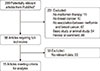 | Figure 1Flow chart of literatures selection for meta-analysis. Flow chart shows literature search for studies in relation to association between metformin and breast cancer. *EMbase was also searched but with no additional articles. |
 | Figure 2Overall pooled relative risk (RR) of studies on incidence of breast cancer. Forest plot shows the association between metformin therapy among women with diabetes and incidence of breast cancer. Weights are from random effects analysis.CI=confidence interval.
|
 | Figure 3Forest plot of overall studies on mortality of breast cancer. Forest plot shows the association between metformin therapy and mortality of breast cancer. Weights are from random effects analysis.RR=relative risk; CI=confidence interval.
|
Table 1
Characteristics of the included studies of metformin and incidence of breast cancer

| Study | Country | Ethnicity | Study design | Comparison | No. of cases or deaths | Population | OR*/HR†/RR‡ | Low CI | Up CI | Adjustments | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tseng et al. (2014) [18] | Taiwan | Asian | CC | None | 191,195 | 443,847 | 0.63 | 0.597 | 0.665 | Age, hypertension, COPD, stroke, nephropathy, ischemic heart disease, peripheral arterial disease, obesity, dyslipidemia, urinary tract disease, statin, fibrate, ACEI/ARB, CCB, sulfonylurea, insulin, acarbose, pioglitazone, rosiglitazone, aspirin, ticlopidine, clopidogrel, and NSAIDs | 8 |
| Qiu et al. (2013) [19] | U.K. | Caucasian | CC | Sulfonylureas | 39,070 | 16,904 | 1.04 | 0.83 | 1.31 | Age | 7 |
| Redaniel et al. (2012) [21] | British | Caucasian | RC | Other drugs | 151 | 873 | 1.04 | 0.79 | 1.37 | Age, period, region, BMI, year of diagnosis, and weighted HbA1c | 8 |
| Hsieh et al. (2012) [22] | Taiwan | Asian | CC | Sulfonylurea | 2,048 | 2,804 | 1.765 | 1.03 | 3.024 | Age | 5 |
| Chlebowski et al. (2012) [20] | U.S. | All | RC | Other drugs | 104 | 556 | 0.75 | 0.57 | 0.99 | Age, benign breast disease, parity, age at first birth, education, No. of months of breastfeeding, smoking, alcohol consumption, BMI, physical activity, duration of use of estrogen or progesterone, bilateral oophorectomy, and mammogram within 2 years of baseline | 9 |
| Morden et al. (2011) [23] | U.S. | African American | RC | Other drugs | NA | 15,286 | 1.28 | 1.05 | 1.57 | Age, race/ethnicity, diabetes complications, obesity diagnosis, oral estrogen use, Part D low-income subsidy, 14 Charlson comorbidities, and tobacco exposure diagnosis | 7 |
| Bosco et al. (2011) [25] | Denmark | All | CC | Other drugs | 96 | 1,154 | 0.87 | 0.61 | 1.25 | Complications due to diabetes, clinical obesity, age at index date, postmenopausal hormone use, and multiple imputation to impute missing parity | 8 |
| Bodmer et al. (2010) [24] | U.K. | Caucasian | CC | Sulfonylureas | 55 | 172 | 1.4 | 0.94 | 2.09 | Age, sex, general practice, calendar time by matching, use of prandial glucose regulators, acarbose, thiazolidinediones, estrogens, smoking, BMI, diabetes duration, and A1c | 7 |
| Bodmer et al. (2010) [24] | U.K. | Caucasian | CC | Sulfonylureas | 64 | 253 | 0.97 | 0.67 | 1.42 | Age, sex, general practice, calendar time by matching, use of prandial glucose regulators, acarbose, thiazolidinediones, estrogens, smoking, BMI, diabetes duration, and A1c | 7 |
| Bodmer et al. (2010) [24] | U.K. | Caucasian | CC | Sulfonylureas | 11 | 88 | 0.42 | 0.21 | 0.87 | Age, sex, general practice, calendar time by matching, use of prandial glucose regulators, acarbose, thiazolidinediones, estrogens, smoking, BMI, diabetes duration, and A1c | 7 |
| Tsilidis et al. (2014) [26] | U.K. | Caucasian | RC | Other drugs | NA | 95,820 | 1.04 | 0.82 | 1.32 | Smoking status, BMI, alcohol consumption, use of aspirin or NSAIDs, statins, diabetes duration, and year of first antidiabetes prescription | 9 |
*OR/†HR/‡RR=odds ratio/hazard ratio/relative risk; CI=confidence interval; NOS=Newcastle-Ottawa Scale; CC=case-control study; COPD=chronic obstructive pulmonary disease; ACEI/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CCB=calcium channel blocker; NSAIDs=nonsteroidal anti-inflammatory drugs; U.K.=United Kingdom; RC=retrospective cohort study; BMI=body mass index; U.S.=United States of America; NA=not available; A1c=HbA1c.
Table 2
Characteristics of the included studies of metformin and mortality of breast cancer

| Study | Country | Ethnicity | Study design | Comparison | No. of cases or deaths | Population | OR*/HR†/RR‡ | Low CI | Up CI | Adjustments | NOS scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xu et al. (2015) [12] | U.S. | Caucasian | RC | Other drugs | 200 | 236 | 0.47 | 0.26 | 0.86 | Age at diagnosis, sex, race, BMI, tobacco use, insulin use, cancer type, and non-cancer Charlson Comorbidity Index | 8 |
| Xu et al. (2015) [12] | U.S. | Caucasian | RC | Other drugs | 363 | 481 | 0.49 | 0.31 | 0.77 | Age at diagnosis, sex, race, BMI, tobacco use, insulin use, cancer type, and non-cancer Charlson Comorbidity Index | 8 |
| Lega et al. (2013) [13] | U.S. | African American | RC | Other durgs | 1,094 | 2,361 | 0.96 | 0.87 | 1.04 | Age, duration of diabetes (years) before breast cancer, ACG comorbidity score, breast cancer treatments within 1 year of diagnosis, and exposure to glucose-lowering drugs before breast cancer (yes/no) | 8 |
| Currie et al. (2012) [14] | U.K. | Caucasian | RC | Other drugs | 4,671 | 24,186 | 0.967 | 0.695 | 1.345 | Age, sex, smoking history, Townsend index of deprivation, Charlson Comorbidity Index, number of primary care contacts, and year of diagnosis | 7 |
| He et al. (2012) [15] | U.S. | All | CC | Nonmetformin | 88 | 65 | 0.47 | 0.24 | 0.9 | NA | 7 |
| El-Benhawy et al. (2014) [16] | U.S. | African American | CC | Nonusers | 25 | 14 | 0.111 | 0.028 | 0.44 | Other factors | 6 |
| Peeters et al. (2013) [17] | Denmark | Caucasian | CC | Nonmetformin | 112 | 508 | 0.74 | 0.58 | 0.96 | Age, Charlson Comorbidity Index, and use of concomitant medication during follow-up, hormone replacement therapy, and statins in the past 6 months | 7 |
Notes
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.

2. Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014; 16:97–110.

3. Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008; 27:3576–3586.

4. Kim J, Lim W, Kim EK, Kim MK, Paik NS, Jeong SS, et al. Phase II randomized trial of neoadjuvant metformin plus letrozole versus placebo plus letrozole for estrogen receptor positive postmenopausal breast cancer (METEOR). BMC Cancer. 2014; 14:170.

5. Oppong BA, Pharmer LA, Oskar S, Eaton A, Stempel M, Patil S, et al. The effect of metformin on breast cancer outcomes in patients with type 2 diabetes. Cancer Med. 2014; 3:1025–1034.

6. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

7. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute;c2014. Accessed January 19th, 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
8. Wang T. The link between Parkinson's disease and breast and prostate cancers: a meta-analysis. Int J Neurosci. 2014; 12. 18. Epub. DOI: 10.3109/00207454.2014.986265.

9. Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychol Methods. 1998; 3:354–379.

10. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

11. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

12. Xu H, Aldrich MC, Chen Q, Liu H, Peterson NB, Dai Q, et al. Validating drug repurposing signals using electronic health records: a case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc. 2015; 22:179–191.

13. Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013; 36:3018–3026.

14. Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012; 35:299–304.

15. He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012; 23:1771–1780.

16. El-Benhawy SA, El-Sheredy HG. Metformin and survival in diabetic patients with breast cancer. J Egypt Public Health Assoc. 2014; 89:148–153.

17. Peeters PJ, Bazelier MT, Vestergaard P, Leufkens HG, Schmidt MK, de Vries F, et al. Use of metformin and survival of diabetic women with breast cancer. Curr Drug Saf. 2013; 8:357–363.

18. Tseng CH. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res Treat. 2014; 145:785–790.

19. Qiu H, Rhoads GG, Berlin JA, Marcella SW, Demissie K. Initial metformin or sulphonylurea exposure and cancer occurrence among patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013; 15:349–357.

20. Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012; 30:2844–2852.

21. Redaniel MT, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer Causes Control. 2012; 23:1785–1795.
22. Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012; 2012:413782.

23. Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. 2011; 34:1965–1971.

24. Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010; 33:1304–1308.
25. Bosco JL, Antonsen S, Sørensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011; 20:101–111.

26. Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014; 37:2522–2532.

27. Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. 2014; 35:2035–2045.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download