Abstract
Purpose
Histone deacetylase 6 (HDAC6) is an enzyme that deacetylates heat-shock protein 90 (HSP90). Many studies have investigated the role of HDAC6 and HSP90 in tumorigenesis and in the prognosis of cancer patients. This study aimed to evaluate the prognostic value of HDAC6 and acetylated HSP90 (acetyl-HSP90) in a cohort of breast cancer patients.
Methods
Immunohistochemical analysis of 314 surgical specimens obtained from patients with invasive breast cancer was carried out to assess standard pathologic factors and the expression of HDAC6 and acetyl-HSP90. Statistical analyses were performed to determine the association between HDAC6, acetyl-HSP90, and conventional clinicopathological factors, and the prognostic values of these factors were evaluated.
Results
HDAC6 expression did not show any correlation with other clinicopathological factors, but acetyl-HSP90 was significantly correlated with histologic grade (p=0.001) and the Ki-67 index (p=0.015). HDAC6 and acetyl-HSP90 expression were significantly associated with each other (p=0.047). Although HDAC6 was not prognostic for disease-free survival (DFS), some patients with high expression of HDAC6 experienced recurrence 5 years after diagnosis, while there was no recurrent disease after 5 years in those with low expression. Acetyl-HSP90 was significantly associated with the DFS of all patients (p=0.016) and with high HDAC6 expression (p=0.017), but not with low expression.
Many clinicopathological factors such as age at diagnosis, tumor size, nodal metastasis, histologic grade, estrogen receptor (ER) and progesterone receptor (PR) expression, and human epidermal growth factor receptor 2 (HER2) amplification are known to be associated with the prognosis of breast cancer patients. A combination of surgical resection, systemic therapy, and radiation therapy, based on patients' risk, has significantly improved the prognosis of these patients. However, as it is difficult to predict prognosis and treatment outcome, many studies have tried to deepen our understanding of the molecular events associated with breast cancer development [1234].
Histone deacetylase 6 (HDAC6) has been shown to be involved in the development of various cancers and is thought to be a potential new target for cancer therapy [567]. In breast cancer, HDAC6 has been demonstrated to be a late-responsive estrogen-induced gene [8]. However, the prognostic value of HDAC6 has not yet been clearly identified and the results of previous studies are contradictory. In some studies on ER-positive breast cancer, HDAC6 expression has been associated with a better survival probability and more favorable response to endocrine treatment [89]. Conversely, in another study that investigated the expression of this estrogen-regulated gene, HDAC6-positive patients had a poorer prognosis compared to HDAC6-negative patients [10]. Finally, it has been demonstrated that ER-positive breast cancer cells with high HDAC6 expression had increased cell motility, which suggests that these cells had more aggressive features resulting in a poorer prognosis [11].
Heat-shock protein 90 (HSP90) is a molecular chaperone that regulates the conformation and functions of client proteins from various cellular processes including the cell cycle and cell survival [1213]. It is well known that aberrant expression of HSP90 is associated with a poor prognosis for breast cancer patients, and HSP90 inhibition could be an effective anticancer therapy [131415]. HSP90 is one of the substrates of HDAC6, and it has been shown that inhibition of HDAC6 led to the hyperacetylation and loss of function of HSP90 [16]. Furthermore, previous studies have reported that inhibition of HDAC6 led to antileukemic activity via hyperacetylation of HSP90, which caused the degradation of oncoproteins [1718]. In a recent study, inhibition of HDAC6 by carbamazepine disrupted the function of HSP90 and resulted in the degradation of HER2 in breast cancer [19].
In this study, we evaluated the association between the expression levels of HDAC6 and acetyl-HSP90 in early-stage breast cancer specimens, and also investigated the prognostic values of these molecular markers.
Tissue specimens from 314 patients who underwent surgical resection for invasive breast cancer at Seoul National University Bundang Hospital, between May 2003 and December 2006, were collected. Tissue microarrays were constructed as previously described [20]. Early-stage patients with stage I and II tumors were included. Twenty-three patients who received neoadjuvant chemotherapy were excluded from this study to eliminate any compounding effects of the chemotherapy on the pathologic characteristics. Fifty-eight patients with advanced-stage disease were also excluded. A total of 233 specimens were included in the analysis and their medical records were reviewed. All patients were treated according to the standard clinical guidelines after surgical resection. Informed consent was obtained from patients undergoing pathologic evaluation and tissues samples were obtained in accordance with a protocol approved by the Institutional Review Board (B-0909/083-002).
Immunohistochemical (IHC) staining of HDAC6 and acetyl-HSP90, in addition to the standard prognostic markers such as ER and PR expression, the Ki-67 index, and p53 overexpression, were carried out as described in a previous study [21]. Ki-67 staining >10% was scored as positive. Fluorescence in situ hybridization was performed to assess HER2 amplification in all specimens.
Polyclonal anti-HDAC6 (Abcam, Cambridge, UK) and monoclonal anti-HSP90 (StressGen, Victoria, Canada) were used in IHC for HDAC6 and acetyl-HSP90, respectively. The staining results of the standard markers were interpreted by a breast pathologist, as previously described [21]. Because large sections of the tumor cells expressed HDAC6 and acetyl-HSP90, the most intense signals were evaluated and given intensity scores ranging from 0 (negative) to 3 (strong). These were further categorized into low-expression (0-1) and high-expression (2-3) groups.
The primary endpoint of this study was disease-free survival (DFS), defined as the time measured from the date of diagnosis to the date of locoregional recurrence, distant metastasis, or death. Statistical analyses were performed with SPSS software version 18.0 (SPSS Inc., Chicago, USA). The chi-square test and Fisher exact test were used to assess the association of HDAC6 and acetyl-HSP90 with other clinicopathological factors. The actuarial survival rates were calculated by using the Kaplan-Meier method, and the differences were verified with the log-rank test. The Cox proportional hazards model was used to test the independent prognostic value of each variable in multivariate analysis.
The characteristics of the patients and tumors are summarized in Table 1. Two male breast cancer patients were included, and the median age at diagnosis was 49 years (range, 26-87 years). Of the 233 patients, 224 (96.1%) were diagnosed with invasive ductal carcinomas and 127 (54.5%) had low-grade (grade 1-2) tumors. T1 tumors were present in 137 patients (58.8%) and 152 patients (65.2%) had no lymph node metastasis. ER and PR were positive in 163 patients (70.0%) and 141 patients (60.5%), respectively. HER2 amplification was observed in 35 patients (15.0%).
The median follow-up duration was 6.95 years (range, 0.15-9.99 years). High and low HDAC6 expression levels were found in 148 patients (65.2%) and 79 patients (34.8%), respectively. In addition, high and low acetyl-HSP90 expression levels were found in 105 patients (46.7%) and 120 patients (53.3%), respectively (Figure 1).
Table 2 shows the correlation between HDAC6 and acetyl-HSP90, and other clinicopathological factors. HDAC6 was not associated with pathologic factors such as histologic grade (p=0.086) and HER2 amplification (p=0.078). Acetyl-HSP90 showed a significant correlation with histologic grade (p=0.001) and the Ki-67 index (p=0.015), and a marginally significant correlation with ER positivity (p=0.059). The association between HDAC6 and acetyl-HSP90 is shown in Table 3. Patients with low HDAC6 expression were more likely to have tumors with low acetyl-HSP90 expression (p=0.047).
The results of the univariate and multivariate analyses of DFS are summarized in Table 4. Neither the conventional clinicopathological factors nor HDAC6 were significantly correlated with DFS. Only acetyl-HSP90 was significantly associated with DFS (p=0.016), and this remained significant following multivariate analysis (p=0.049).
Although HDAC6 was not a prognostic predictor of DFS, as shown in Figure 2, Kaplan-Meier survival graphs began to separate after 5 years according to HDAC6 expression levels. While there were no events after 5 years in the HDAC6 low-expression group, the DFS probability of the HDAC6 high-expression group continued to decline. Moreover, the separation of survival graphs was more prominent in the ER-positive group, than in the ER-negative group.
In subgroup analysis, acetyl-HSP90 showed a significant association with DFS in the HDAC6 high-expression group, but not in the low-expression group (Figure 3).
HDACs belong to a large family of enzymes involved in the deacetylation of histone proteins and various cellular proteins such as transcription factors. HDAC is categorized into four classes (I-IV) based on sequence homology [22]. HDAC6 is a member of HDAC class II and contains two homologous deacetylase domains that are thought to contribute independently to overall activity. Unlike other HDACs, HDAC6 is mainly found in the cytoplasm, and it deacetylates nonhistone proteins such as α-tubulin, HSP90, and cortactin. By modulating these substrates, HDAC6 plays an essential role in various cellular processes, including cell motility, cell signaling pathways, and cell survival. Recently, it has been shown that HDAC6 is also associated with the aggresome pathway, which eliminates toxic proteins [323].
In this study, HDAC6 was not a significant prognostic predictor of DFS in patients. Previous studies investigated the association of HDAC6 with various diseases, and evidence that HDAC6 is involved in the process of malignant transformation is increasing. Thus, HDAC6 is thought to be a putative target for anticancer treatment and studies involving HDAC inhibitors are underway [5]. However, reports on the prognostic value of HDAC6 are conflicting. Several studies have illustrated an association between the expression of HDAC6 and the prognosis of various cancers, particularly breast cancer, and they have shown that HDAC6 gene was estrogen-responsive [624]. However, a study by Saji et al. [9] reported no survival difference when HDAC6-positive and -negative groups were compared, but found a better prognosis for the HDAC6-positive group of patients who had ER-positive tumors. Carcinogenesis is a complicated process that involves diverse cellular pathways, which are extensively interconnected. Moreover, because HDAC6 is involved in various processes, HDAC6 expression alone could not be a strong predictor of overall prognosis. Nevertheless, it is evident that HDAC6 is involved in cell motility and invasion of tumor cells in breast cancer, and these features contribute to the aggressiveness of tumor cells and result in a poor prognosis [911]. Therefore, although not predictive of overall prognosis, HDAC6 may be a potential marker of prognosis in certain subsets of patients, and our results support this. Remarkably, the survival graphs began to separate after 5 years of follow-up according to HDAC6 expression levels. Late recurrence 5 years after diagnosis occurred only in the HDAC6 high-expression group, while the graphs plateaued after 5 years in the low-expression group. Based on this result, we speculated that HDAC6 might be a potential predictor of late recurrence, and patients with high HDAC6 expression might need to be followed up more closely and for a longer duration compared to other patients. However, our results are not statistically significant and there are no other published studies with similar data. Therefore, a larger study with longer follow-up is required to examine this further.
In addition, survival graphs, according to HDAC6 expression, separated more prominently for ER-positive than for ER-negative patients. Hayashi and Yamaguchi [25] identified HDAC6 as an estrogen-regulated gene, and showed the association between HDAC6 and the prognosis of breast cancer patients treated with tamoxifen. At present, the ER and PR statuses are used to predict the responsiveness of breast cancer patients to endocrine therapy, but this is not effective for some patients. Many efforts have been made to find better markers to predict the efficacy of endocrine therapy, and there is increasing evidence suggesting an association between HDAC6 expression and responsiveness to endocrine therapy [826]. Our results suggest a possible role for HDAC6 as such a predictive marker, although further investigation is needed.
HSP90 is known to be one of the client proteins of HDAC6. HDAC6 deacetylates HSP90, and when HDAC6 is inhibited, HSP90 becomes acetylated and loses its chaperone function [18]. High expression of HSP90 is known to be a poor prognostic factor in breast cancer [1521], and we had comparable results. In our study, patients with a low level of acetyl-HSP90 showed a poorer prognosis than those with high expression. In multivariate analysis, acetyl-HSP90 was a prognostic predictor of DFS confirming that HSP90 is a strong prognostic factor.
In subgroup analysis, the DFS graphs according to acetyl-HSP90 expression were more separated in the HDAC6 high-expression group than in the low-expression group. A previous study reported that deacetylation of HSP90 by HDAC6 protects various oncoproteins from degradation and suggested a possible role for HDAC6-HSP90 interplay in malignant transformation [27]. In addition, Kovacs et al. [16] reported that HDAC6-dependent HSP90 acetylation regulates the maturation of the glucocorticoid receptor (GR), which has both an antiproliferative and antiapoptotic role. Additionally, a recent study by Abduljabbar et al. [28] evaluated GR expression in breast cancer and reported that it is associated with a favorable outcome in ER-positive breast cancer. Because HDAC6-HSP90 interplay is complex, it may have different effects depending on the specific situation. Therefore, the effect of HDAC6 and HSP90 on the prognosis of cancer patients may not be straightforward. However, our results support the significance of HDAC6-HSP90 interplay in predicting the prognosis of breast cancer patients. Further studies to clarify their roles in different situations are needed.
Our study has several limitations. First, none of the conventional prognostic factors showed significant prognostic value for DFS. Because our study included only early-stage breast cancer patients, the outcome of patients was excellent, and only 19 of 233 cases (8.2%) experienced recurrence or death. Therefore, many clinicopathological factors such as patient age, histologic grade, hormone receptor status, and HER2 amplification, known as powerful prognostic factors, were not predictive in our patients due to the low recurrence rate. For the same reason, the prognostic power of HDAC6 and acetyl-HSP90 may have been underestimated. Therefore, a further study with a larger number of patients and longer follow-up period may indicate a more significant prognostic value for HDAC6 and acetyl-HSP90.
Second, because HDAC6 and HSP90 are new molecules that are involved in cancer pathways, there are no standard methods for IHC staining or scoring systems for these molecules. Therefore, this precludes a direct comparison of our results and those obtained in other studies.
In conclusion, we demonstrated a correlation between HDAC6 and acetyl-HSP90, and investigated the prognostic value of HDAC6 and acetyl-HSP90 in early-stage breast cancer patients. Our results contribute to a more accurate prediction of breast cancer prognosis using HDAC6 and acetyl-HSP90, and suggest that a new cancer therapy targeting HDAC6 and HSP90 could be implemented in the future to improve the treatment outcome of breast cancer patients.
Figures and Tables
Figure 1
Immunohistochemical analyses of histone deacetylase 6 (HDAC6) and acetylated heat-shock protein 90 (acetyl-HSP90) (×400). (A) Negative for HDAC6. (B) Positive for HDAC6. (C) Negative for acetyl-HSP90. (D) Positive for acetyl-HSP90.
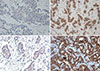
Figure 2
Kaplan-Meir survival curves for disease-free survival of breast cancer patients based on histone deacetylase 6 expression in total patients (A), estrogen receptor negative (B), and ER-positive (C) group.
HDAC6=deacetylase 6; ER=estrogen receptor.

Figure 3
Kaplan-Meir survival curves for disease-free survival of breast cancer patients based on acetylated heat-shock protein 90 (acetyl-HSP90) level in total patients (A), histone deacetylase 6 (HDAC6) low expression (B), and HDAC6 high expression (C) group.

Table 1
Clinicopathological characteristics

Table 2
Correlation of clinicopathological factors with HDAC6 expression and HSP90 acetylation

Table 3
Expression of HDAC6 and acetylated HSP90

| HDAC6 expression | Acetyl-HSP90 expression | p-value | |
|---|---|---|---|
|
Low No. (%) |
High No. (%) |
||
| Low | 49 (63.6) | 28 (36.4) | 0.047 |
| High | 70 (49.0) | 73 (51.0) | |
Table 4
Univariate and multivariate analysis for disease-free survival

References
1. Cai FF, Kohler C, Zhang B, Wang MH, Chen WJ, Zhong XY. Epigenetic therapy for breast cancer. Int J Mol Sci. 2011; 12:4465–4487.

2. Nwabo Kamdje AH, Seke Etet PF, Vecchio L, Tagne RS, Amvene JM, Muller JM, et al. New targeted therapies for breast cancer: a focus on tumor microenvironmental signals and chemoresistant breast cancers. World J Clin Cases. 2014; 2:769–786.

3. Duong V, Bret C, Altucci L, Mai A, Duraffourd C, Loubersac J, et al. Specific activity of class II histone deacetylases in human breast cancer cells. Mol Cancer Res. 2008; 6:1908–1919.

4. Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004; 15:651–662.

5. Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011; 2011:875824.

6. Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008; 68:7561–7569.

7. Yoshida M, Furumai R, Nishiyama M, Komatsu Y, Nishino N, Horinouchi S. Histone deacetylase as a new target for cancer chemotherapy. Cancer Chemother Pharmacol. 2001; 48:Suppl 1. S20–S26.

8. Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004; 10:6962–6968.

9. Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005; 24:4531–4539.

10. Yoshida N, Omoto Y, Inoue A, Eguchi H, Kobayashi Y, Kurosumi M, et al. Prediction of prognosis of estrogen receptor-positive breast cancer with combination of selected estrogen-regulated genes. Cancer Sci. 2004; 95:496–502.

11. Rey M, Irondelle M, Waharte F, Lizarraga F, Chavrier P. HDAC6 is required for invadopodia activity and invasion by breast tumor cells. Eur J Cell Biol. 2011; 90:128–135.

12. Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003; 14:1169–1176.

13. Whitesell L, Lin NU. HSP90 as a platform for the assembly of more effective cancer chemotherapy. Biochim Biophys Acta. 2012; 1823:756–766.

14. Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992; 51:613–619.

15. Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007; 67:2932–2937.

16. Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005; 18:601–607.

17. Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, et al. HDAC6 inhibition enhances 17-AAG: mediated abrogation of Hsp90 chaperone function in human leukemia cells. Blood. 2008; 112:1886–1893.

18. Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005; 280:26729–26734.

19. Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011; 348:165–171.

20. Hyun CL, Lee HE, Kim KS, Kim SW, Kim JH, Choe G, et al. The effect of chromosome 17 polysomy on HER-2/neu status in breast cancer. J Clin Pathol. 2008; 61:317–321.

21. Song CH, Park SY, Eom KY, Kim JH, Kim SW, Kim JS, et al. Potential prognostic value of heat-shock protein 90 in the presence of phosphatidylinositol-3-kinase overexpression or loss of PTEN, in invasive breast cancers. Breast Cancer Res. 2010; 12:R20.

22. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006; 6:38–51.
23. Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013; 280:775–793.
24. Inoue A, Yoshida N, Omoto Y, Oguchi S, Yamori T, Kiyama R, et al. Development of cDNA microarray for expression profiling of estrogen-responsive genes. J Mol Endocrinol. 2002; 29:175–192.

25. Hayashi S, Yamaguchi Y. Estrogen signaling and prediction of endocrine therapy. Cancer Chemother Pharmacol. 2005; 56:Suppl 1. 27–31.

26. Sabnis GJ, Goloubeva O, Chumsri S, Nguyen N, Sukumar S, Brodie AM. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res. 2011; 71:1893–1903.
27. Krämer OH, Mahboobi S, Sellmer A. Drugging the HDAC6-HSP90 interplay in malignant cells. Trends Pharmacol Sci. 2014; 35:501–509.

28. Abduljabbar R, Negm OH, Lai CF, Jerjees DA, Al-Kaabi M, Hamed MR, et al. Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res Treat. 2015; 150:335–346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download