Abstract
Purpose
The unmanageable side effects caused by current chemotherapy regimens to treat cancer are an unresolved problem. Although many phytonutrients are useful as chemoprevention without side effects, their effects are slower and smaller than conventional chemotherapy. In the present work, we examined the cumulative effect of two phytonutrients, curcumin and citral, on breast cancer cell lines and compared their effect with the known chemotherapy regimen of cyclophosphamide, methotrexate, and 5-fluorouracil.
Methods
Using cultured breast cancer and normal epithelial cells, the cytotoxic and apoptotic effect of curcumin and citral was evaluated in vitro. The synergistic effect of curcumin and citral was calculated by a combination index study using the method by Chou and Talalay. Cell death pathways and mechanisms were analyzed by measuring intracellular reactive oxygen species (ROS) and apoptotic protein levels.
Results
Curcumin and citral caused dose and time dependent cell death and showed a synergistic effect at effective concentration EC50 and above concentrations in breast cancer cells without disturbing normal breast epithelial cells. With combination curcumin and citral treatment, apoptosis induction and cell cycle arrest at G0/G1 phase in breast cancer cells were observed. Curcumin and citral generated ROS and activated p53 and poly (ADP-ribose) polymerase-1 mediated apoptotic pathways.
Many natural dietary compounds have chemoprevention effects by blocking, inhibiting, reversing, or retarding the process of carcinogenesis [1]. Nutritional factors may also enhance the anticancer effects of chemotherapy and may be useful for the management of certain chemotherapy induced side effects Curcumin, an active component of turmeric derived from the plant Curcuma longa, has been widely employed as a medicine since ancient times in India and other parts of Southeast Asia. Curcumin has anti-inflammatory, antioxidant, and chemopreventive properties and chemotherapeutic potential with no apparent side effects [2]. Curcumin was shown to inhibit the proliferation of breast cancer cells in vivo and in vitro [34]. Citral, a key component of essential oils from lemon grass (Cymbopogan citrates), exhibits antimicrobial, antifungal, antioxidant, and free radical scavenging activities in mice [5]. In addition, citral was shown to have an antimutagenic effect in cyclophosphamide induced mutagenicity [6]. Although curcumin and citral have been assessed independently as chemoprevention agents, the use of curcumin and citral in combination and their side effects profile as chemotherapy has not been studied.
Even though most anticancer agents or drugs initially induce reactive oxygen species (ROS) generation to kill cancer cells [78], the cellular mechanisms underlying generation of ROS remain unclear. It is not known whether ROS generation is the only way to induce cancer cell death, but it is apparent that ROS generation plays a major role in inducing apoptosis. It has been demonstrated that production of ROS may be the cause of tumor cell apoptosis as a result of curcumin treatment [9]. However, curcumin has been also shown to be an antioxidant and a free radical scavenger that inhibits the ability of chemotherapeutic drugs to induce apoptosis [10]. Curcumin was found to suppress multiple signaling pathways [3], whereas citral was shown to induce caspase-3 mediated apoptosis [1112].
In the present study, we assessed the cytotoxicity of classical cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) combinational chemotherapy on breast cancer cell lines (MCF 7 and MDA MB 231) and a normal breast epithelial cell line (MCF 10A). The dose and time dependent effects of combination curcumin and citral treatment on breast cancer and normal cell lines were also studied and compared to the CMF regimen. Since systemic toxicity is a major limitation of chemotherapy, these phytonutrients could be developed as an alternative therapy for the treatment of cancer.
The MCF 7 and MDA MB 231 cell lines were purchased from the National Centre for Cell Services (Pune, India) and were cultured in Dulbecco's Modified Eagle's Medium (DMEM) and Leibovitz's L-15, respectively, supplemented with 10.0% fetal bovine serum (FBS) in 5.0% CO2 at 37℃. MCF 10A cell line was kindly gifted by Dr. Annapoorni Rangarajan (Department of Molecular Reproduction, Development and Genetics, Indian Institute of Science, Bangalore, India) and was cultured in DMEM/F-12 supplemented with 10.0% FBS, 0.5 µg/mL of hydrocortisone, 10 µg/mL of insulin, 20 ng/mL of epidermal growth factor, 0.5 KU/mL of penicillin, 0.1 mg/mL of streptomycin, and 0.5 µg/mL of amphotericin B in 5.0% CO2 at 37℃. Animal cell culture grade chemicals and solutions were purchased from Himedia (Ahmedabad, India). DMEM, Leibovitz's L-15, DMEM/F-12, FBS, curcumin, citral, 3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT), 4'6-diamidino-2-phenylindole (DAPI), ethidium bromide, propidium iodide (PI), and 2',7'-dichlorodihydrofluorescein diacetate (DCFHDA) were purchased from Sigma (St. Louis, USA).
To screen the survival of MCF 7 and MDA MB 231 cells treated with curcumin and citral, the clonogenic survival assay was performed. Approximately 800 to 1,000 cells of MCF 7, MDA MB 231, and MCF 10A (control) were seeded in six well plates and grown for 24 hours. Thereafter, cells were treated with different concentrations of curcumin (0.0-80 µM) and citral (0.0-160 µM) and allowed to grow for 24 hours. The medium was replaced with fresh medium and allowed to grow up to five or six doublings. The medium was removed after colony formation, and plates were allowed to air dry. The colonies were stained with 0.2% crystal violet and counted using gel documentation system, AlphaDigiDoc-RT (J. H. Bio Innovations Pvt. Ltd., Bangalore, India). Experiments were conducted in triplicate, and the data were presented as percent survival compared to untreated cells.
The MTT assay was carried out to measure cell viability of MCF 7, MDA MB 231, and MCF 10A. Approximately 1×104 cells were grown for 24 hours and then treated with increasing concentrations of cyclophosphamide (0-20 mM), methotrexate (0-20 mM), 5-fluorouracil (0-20 mM), curcumin (0-160 µM), or citral (0-400 µM). The cells were incubated for 24, 48, and 72 hours, and cell viability was measured by MTT assay [13]. All experiments were performed in triplicate, and the data are presented as percent viability and compared to untreated cells whose percent viability was considered 100%. Once percent viability was obtained, the drug response curve was generated, and effective concentration (EC50) was measured using software MasterPlex 2010 (http://download.cnet.com/MasterPlex-2010/3000-2054_4-75373446.html). Combination curcumin and citral treatment was evaluated by calculating the Combination Index (CI) value using the CalcuSyn software from Biosoft (Cambridge, UK), with the method used by Chou and Talalay [14]. In this analysis, synergy was defined as a CI <1.0, antagonism as a CI >1.0, and additivity as CI values not significantly different from 1.0.
Annexin V-fluorescein isothiocyanate (FITC) staining was done in conjunction with PI in order to distinguish various stages of apoptotic cells. The MCF 7 cells were treated with curcumin (40 µM), citral (80 µM), and CMF (3.5, 0.75, 1.5 mM); and annexin V/PI staining analysis was performed using the Calbiochem annexin V-FITC apoptosis detection kit from Calbiochem (San Diego, USA) according to the manufacturer's protocol. Cells were examined using fluorescence microscopy (520/620 nm) (Olympus BX41; Olympus, Center Valley, USA). The distinction was made between early apoptotic cells (annexin V-positive, PI-negative), late apoptotic/necrotic cells (annexin V-positive, PI-positive), viable cells (annexin V-negative, PI-negative), and dead cells (annexin V-negative, PI-positive).
Apoptosis was further confirmed by the terminal deoxyribonucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay in curcumin (40 µM) and citral (80 µM) treated MCF 7 cells for 24, 48, and 72 hours. TUNEL detects the DNA fragmentation that is the characteristic of apoptosis. TUNEL assay was performed using the TUNEL assay kit from Calbiochem, and the protocol was followed as per the manufacturer's guideline. The cells were evaluated using fluorescence microscopy (330-380 nm) to identify TUNEL positive cells, and the results are presented as percent of apoptotic cells.
The DNA damage in the curcumin and citral treated MCF 7 cells were analyzed by COMET assay. Seventy percent confluent cells treated with curcumin (40 µM), citral (80 µM), or 100 µM H2O2 (a positive control) for 24 hours were harvested, washed with phosphate buffered saline (PBS), and resuspended in ice-cold PBS. COMET assay was performed according to the protocol described previously [15]. Staining of DNA in the gel was carried out by ethidium bromide. DNA damage was visualized using fluorescence microscopy (excitation at 510-550 nm and emission at 590 nm), and the numbers of COMET were recorded. The COMET parameters were analyzed by TriTek CometScore™ software (Tritek Corp., Sumerduck, USA).
The effect of curcumin and citral either independently or in combination on cell cycle arrest was analyzed by flow cytometry. The cells treated with curcumin (40 µM), citral (80 µM), and in combination were stained with PI, and the proportion of cells in various phases of the cell cycle was monitored. The protocol was performed as described previously [13]. The DNA content of stained cells was analyzed using Cell Lab Quanta software from Beckman Coulter (Fullerton, USA).
Approximately 70% confluent MCF 7 and MCF 10A cells were treated with drugs, curcumin, and citral in triplicate and incubated for 4 hours. DCFHDA was subsequently added at a final concentration of 30 µM to two of the three wells treated with agents and incubated for 40 minutes. After incubation, fluorescence was read at 485 nm excitation and 535 nm emission in a fluorescence plate reader (Hitachi 7000; Hitachi, Tokyo, Japan). The wells containing drugs, curcumin, or citral but not DCFHDA acted as blanks for each sample. ROS production was expressed as percent increase in fluorescence relative to untreated control cells and presented as final percent ROS.
Total glutathione (GSH) content was determined by measuring absorbance of standard oxidized glutathione (GSSG) at 412 nm [16]. In a final volume of 0.5 mL, the reaction mixture contained 100 mM phosphate buffer (pH 7.0), 1 mM EDTA, 0.24 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), 0.0756 mM 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), and 0.06 units glutathione reductase (GR). Then, 100 µL of the appropriate GSSG standard or 100 µL of the crude extract (prepared from the cells treated with curcumin, citral, and their combination) was added to each reaction mixture. The absorbance of known concentrations of GSSG was used to generate a standard curve. The experiment was performed in triplicate, and the data are presented as mean with standard deviation (SD).
The endogenous levels of p53 protein, phospho-p53 (Ser15), total Bad, phospho-Bad (Ser112), and cleaved poly (ADP-ribose) polymerase-1 (PARP) in the curcumin (40 µM) or citral (80 µM) treated MCF 7 cells, untreated MCF 7, and MCF 10A cells were detected using CST's PathScan Apoptosis Multi-target Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) kit (Cell Signaling, Beverley, USA), according to the manufacturer's instructions.
Results are presented as mean±SD. Statistical analysis was performed using one-way analysis of variance followed by Tukey's high significant difference test. SPSS software version 17.0 (SPSS Inc., Chicago, USA) was used for the statistical analysis, where a p-value <0.05 was considered significant.
MCF 7 and MDA MB 231 cells were treated with varying concentrations (0.0-20 mM) of chemotherapeutic drugs (cyclophosphamide, methotrexate, 5-fluorouracil) for 24, 48, and 72 hours to determine their EC50 and duration of action. The use of these drugs independently or in combination resulted in a dose dependent decrease in the viability of MCF 7 and MDA MB 231 (breast cancer cells) and MCF 10A (control) cells (Figure 1). The EC50 obtained from the drug response curve of cyclophosphamide, methotrexate, and 5-fluorouracil was 7, 1.5, and 3 mM, respectively, for MCF 7 and 10, 2, and 2.5 mM, respectively, for MDA MB 231 (data not shown). For analysis of the drug combination effect, the chemotherapeutic regimen CMF was used. The combined treatment of CMF showed an EC50 effect at 3.5, 0.75, and 1.5 mM, respectively.
The effect of curcumin and citral on the clonogenic survival of MCF 7, MDA MB 231, and MCF 10A cells was assessed by colony-forming ability and the MTT assay. Curcumin treatment caused a dose-dependent reduction in the colony-forming ability of MCF 7 and MDA MB 231 cells but did not affect normal epithelial breast cells (Figure 2). The EC50 for curcumin and citral was 40 and 80 µM, respectively, for MCF 7 cells and 50 and 100 µM, respectively, for MDA MB 231. Based on this, the concentration range of curcumin and citral used for combination treatment of MCF 7 cells was 10-80 µM and 20-160 µM, respectively.
Combination treatment of curcumin and citral resulted in a dose-dependent decrease in survival of cancer cells, while normal cells were affected less (Figure 3A). Combined treatment of curcumin and citral at EC50 and EC75 in MCF 7 cells showed significant synergism; however, at EC25 it was additive (Figure 3B). The combination of curcumin and citral at their EC50 was found to be synergistic and more efficient relative to CMF treatment. In MDA MB 231, combination curcumin and citral treatment showed significant synergy at EC75 but not at EC50. Combination treatment was additive at EC25. Hence, EC50 concentrations of curcumin and citral were used in subsequent experiments.
Annexin V-FITC conjugated to PI assay was carried out to confirm whether apoptosis was induced by curcumin or citral alone or in combination. An externalization of phospholipid phosphatidylserine in treated cells was visualized by its binding to annexin V, allowing for microscopic detection of apoptotic cells. Annexin V/PI dual staining showed that curcumin and citral induced apoptosis in MCF 7 cells. The number of cells in the early stages of apoptosis was increased significantly in MCF 7 cells treated with 40 µM of curcumin (47.8% vs. 24.6%) and 80 µM of citral (48.5% vs. 22.7%, p<0.05) relative to control (Figure 4A). The number of viable cells significantly decreased from 67.0% to 30.2% with curcumin and from 63.5% to 30.2% with citral as visualized by annexin V and PI staining (p<0.05). Treatment of cells with CMF resulted in 49.8% cell death, which comprised a nonsignificant distribution of cells in the early and late phases of apoptosis. Combination treatment with curcumin and citral at the EC50 concentration led to a significant increase in early apoptotic cells (p<0.01).
The TUNEL assays revealed a dose and time dependent increase in the induction of apoptosis was observed in MCF 7 cells treated with curcumin and citral (Figure 4B). Treatment of MCF 7 cells with curcumin and citral at the EC50 concentration induced a significantly higher percentage of apoptosis than untreated MCF 7 and MCF 10A cells (p<0.01). Combined treatment of curcumin and citral significantly increased the apoptosis percentage compared to CMF treatment (p<0.01). These results confirmed that combination curcumin and control treatment induced apoptosis in MCF 7 cells.
The role of DNA damage in curcumin and citral induced cell death was analyzed in MCF 7 cells by the COMET assay. DNA damage was assessed by measuring the COMET parameters comet tail length, percent DNA in the tail, and tail moment. Curcumin and citral increased DNA damage, as evidenced by an increase in the comet tail lengths with increasing concentration. Combined treatment of curcumin and citral resulted in apparent DNA damage, and this increase in COMET parameters in damaged MCF 7 cells was significantly greater than CMF treatment (p<0.05) (Figure 5).
To determine the effect of curcumin and citral on cell-cycle progression of MCF 7 cells; CMF, curcumin, or citral treated and untreated MCF 7 cells were stained with PI and analyzed by flow cytometry. After 24 and 48 hours of treatment, curcumin arrested MCF 7 cells in the G0/G1 phase. Citral treatment did not arrest cells in a particular phase of cell cycle; rather cells were dispersed in all phases at 24 hours. At 48 hours of incubation, the percentage of cells in the G0/G1 phase was significantly increased from 39.4% to 65.7% and the percentage of cells in the G2 phase decreased from 39.2% to 21.1% (p<0.05). The combined treatment of curcumin and citral significantly increased cells arrested in the G0/G1 phase at 24 and 48 hours compared to either treatment alone (p<0.05) (Figure 6).
ROS production was measured using DCHFDA in cancer (MCF 7) and normal (MCF 10A) cells treated with drugs, curcumin, and citral. It was observed that untreated cancer cells generated higher ROS than normal cells (p<0.05). CMF treatment significantly increased ROS production in both cancer cells (p<0.001) and normal cells (p<0.01) compared to untreated cells. Curcumin and citral also induced ROS production in MCF 7 cells (Figure 7A). Combined treatment of curcumin and citral significantly reduced ROS production as compared to CMF treatment in MCF 7 cells (p<0.05).
Compared to normal cells (MCF 10A), the total GSH content was significantly higher in untreated MCF 7 cells (p<0.05). Upon treatment with CMF, total GSH content was significantly increased compared to untreated MCF 7 cells (p<0.05). An increase in total GSH content was also observed at the EC50 concentration of curcumin and citral. Combined treatment of curcumin and citral significantly decreased the GSH level compared to CMF treatment (p<0.01) (Figure 7B).
To detect activation of the apoptosis pathway, endogenous levels of proapoptotic, apoptotic, and antiapoptotic proteins in MCF 7 cells treated with curcumin and citral and untreated MCF 10A cells were measured. The level of phosphorylated p53 was significantly lower in the MCF 7 cells treated with curcumin and citral at their EC50 concentration than untreated MCF 7 and MCF 10A cells (Figure 8). The level of total p53 was significantly higher in curcumin and citral treated cancer cells (p<0.05). The level of phosphorylated Bad was higher in untreated cancer and normal cells than curcumin and citral treated cells. A dramatic increase in the level of total Bad was observed in cells treated with curcumin, suggesting activation of apoptosis. The rise in total Bad level was significantly higher in untreated MCF 7 cells and MCF 10A cells than citral treated cells (p<0.05). The level of cleaved PARP was significantly different between cells treated with curcumin or citral and untreated cancer cells and normal cells (p<0.05).
In the present study, we identified effective concentrations for the antiproliferative effects of curcumin and citral alone or in combination. In addition, we compared the effects of curcumin and citral to CMF on breast cancer cell lines (MCF-7 and MDA MB 231) and provided insight into the underlying mechanisms involved.
Initially, when each chemotherapeutic drug, curcumin, and citral were tested independently to examine efficacy and cytotoxicity, we found that drug treatment dramatically decreased the proliferation of MCF 7 and MDA MB 231 cells, especially at 48 and 72 hours, without affecting normal breast epithelial cells (MCF 10A). When the CMF combination treatment was carried out, a two-fold decrease in EC50 was observed relative to each drug alone, suggesting that the combination therapy is a better treatment option.
Phytonutrients affect the cell cycle by inhibiting proliferation and/or inducing apoptotic death in cancer cells [17]. Such modulatory effects of curcumin and citral on MCF 7, MDA MB 231, and MCF 10A cells were observed in the present study. As a measure of cytotoxicity, we found that curcumin and citral dose and time dependently increased cell death in breast cancer cells but not normal cells. These results are in accordance with other studies that demonstrated that curcumin and citral inhibited cell proliferation and caused cytotoxicity in many cancer cell lines, including breast cancer cell lines [318]. As was done for chemotherapeutic drugs, the EC50 of curcumin and citral was calculated. In MCF 7 cells, the EC50 was 40 µM, consistent with the EC50 of curcumin previously reported (between 20 and 80 µM) [19]. For MDA MB 231 cells, the EC50 value of curcumin was reported to be 44 µM [20], whereas in present study the EC50 was 50 µM. The EC50 of citral in MCF 7 cells was 80 µM, whereas it was previously reported to be 180 µM [18]. The EC50 of citral in MDA MB 231 had not been reported previously.
When adding a combination of drugs to a cell culture system, the resulting effect can be synergistic, additive, or antagonistic. Since combined treatment may lead to positive interactions that can be useful in cancer therapy, different combinations of curcumin and citral were used and their CIs were calculated. The CI of these combinations at EC50 concentration of curcumin and citral was synergistic in the MCF 7 cell line, demonstrating their potential use as a combination therapy. In MDA MB 231 cells, however, we did not observe significant synergy at EC50 concentrations. The present study is the first to report the CIs of curcumin and citral.
Based on our results of annexin V/PI dual staining, it was concluded that curcumin and citral treated cells underwent apoptosis. This increase in early apoptotic cells in MCF 7 cells with combination curcumin and citral treatment indicated that the use of these two natural compounds together could be a synergistic approach for the treatment of cancer cells. Induction of apoptosis in the MCF 7 cell line by curcumin has been reported previously [32122]. Our study is the first to study the effect of citral in these cells. The treatment of MCF 7 cells with curcumin and citral showed TUNEL positive cells, as demonstrated by the TUNEL assay, which is characteristic of cells undergoing apoptotic cell death. Combination treatment of MCF 7 cells with curcumin and citral resulted in substantial apoptotic phenomenon, supporting the use of curcumin and citral in combination for treating breast cancer. Other apoptotic studies using the TUNEL assay have also shown that curcumin induced apoptosis in MCF 7 cells [2324].
COMET parameters were significantly altered in MCF 7 cells treated with curcumin, citral, or their combination, which is indicative of DNA damage. The extent of DNA damage present in these cells could explain the cytotoxicity observed with this treatment.
Curcumin at the EC50 concentration arrested the cell cycle in the G0/G1 phase at 24 hours, indicating that cells were not entering into the S phase and were unable to replicate, thus leading to cell death. A previous study had similarly shown that curcumin arrested the cell cycle at G1 phase in MCF 7 cells [25]. Citral arrested cell cycles in G0/G1 phase after 48 hours of treatment, suggesting that the cells were in a quenching state at 24 hours and then started arresting in G0/G1 phase, which ultimately led to apoptosis. In contrast to our study, it was previously reported that citral arrested MCF 7 cells in the G2/M phase of the cell cycle [23]. As curcumin and citral both arrested the cell cycle in the G0/G1 phase in a cell cancer line, their use in combination would be a better therapeutic choice than curcumin or citral alone.
Evidence from recent studies suggested that cancer cells, compared to normal cells, are under increased oxidative stress due to oncogenic transformation, alterations in metabolic activity, and increased generation of ROS [26]. In the present study, it was observed that the cancer cells (MCF 7) generated more ROS than normal cells (MCF 10A). As expected, chemotherapeutic drugs also induced oxidative stress, as shown by the high ROS value in MCF 7 cells. Curcumin also caused significantly high ROS generation in the MCF 7 cells, which was consistent with other studies [32125]. This increase in ROS was reflected by the activation of the antioxidant system, as evidenced by an increase in total GSH level.
The effect of curcumin on cell cycle regulatory proteins was examined, and it was observed that curcumin and citral enhanced the expression of tumor suppressor protein p53. Curcumin and citral upregulated the expression of tumor Bcl2 family proapoptotic protein Bad in MCF 7 cells. Furthermore, the execution of apoptosis was increased, as shown by an increased level of cleaved PARP. Other groups have shown that curcumin interferes with multiple cell signaling pathways, including cell cycle and apoptosis [325], and that citral induces caspase-3 activity in MCF 7 and other cell lines [11]. Here, curcumin and citral induced high levels of ROS generation, which led to activation of apoptosis pathway by deactivation of antiapoptotic proteins like phosphorylated p53 and phosphorylated Bad.
In conclusion, the current study demonstrated that combination treatment of curcumin and citral induced apoptosis in MCF 7 cells but not MCF 10A cells and arrested the cell cycle. Thus, our results identified combination curcumin and citral and their synergistic effect as a treatment option for breast cancer.
Figures and Tables
 | Figure 1Survival of MCF 7 and MDA MB 231 cells after exposure to chemotherapeutic drugs. Dose dependent cell viability of MCF 7, MDA MB 231, and MCF 10A cells, after combination treatment with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) was assessed by MTT assay. The concentration of each combination is shown below the graph. Data were plotted as percent viability (% control). At a zero concentration of drugs, % viability was considered 100%. The data represent the mean±standard deviation of one of the three similar experiments each performed in triplicate. |
 | Figure 2Clonogenic survival of breast cancer (MCF 7 and MDA MB 231) and breast epithelial (MCF 10A) cell lines treated with various concentrations of curcumin and citral for 24 hours. Data were plotted as percent viability (% control). At a zero concentration of curcumin and citral, the % viability was considered 100%. The data represent the mean±standard deviation of one of the three similar experiments each performed in triplicate. |
 | Figure 3Combined effect of curcumin (Cur) and citral (Cit) on the survival of MCF 7 and MDA MB 231 cells. (A) The concentration used of Cur1, Cur2, Cur3, and Cur4 was 10, 20, 40, and 80 µM, respectively. Whereas, the concentration used of Cit1, Cit2, Cit3, and Cit4 was 20, 40, 80, and 160 µM, respectively. The concentration of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) was 3.5, 0.75, and 1.5 mM, respectively. Data were plotted as percent viability (% control). At a zero concentration of drugs, curcumin and citral, the % viability was considered 100%. The data represent the mean±standard deviation of one of the three similar experiments each performed in triplicate. (B) Combination Index (CI) of curcumin and citral. The graph shows the mean values of the combination index at the affected fractions of 25.0% (IC25), 50.0% (IC50), and 75.0% (IC75), when curcumin and citral were used in combination in MCF 7 and MDA MB 231 cells. A CI value less than 1 indicates synergism, a CI not different from 1 indicates an addition, and a CI higher than 1 indicates antagonism. The data represent the mean±standard deviation of one of the three similar experiments each performed in triplicate. |
 | Figure 4Induction of apoptosis in MCF 7 cells by curcumin and citral treatment. (A) Annexin V-fluorescein isothiocyanate/propidium iodide (PI) dual staining of the MCF 7 cells exposed to cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), curcumin or citral and to the combination of curcumin and citral. Graph represents the distribution of apoptotic cells based on annexin V/PI staining. The apoptotic cells are stained with annexin V (green fluorescence), and the necrotic cells are stained with PI (red fluorescence). The image showed the stained cells treated with curcuminn (40 µM) and citral (80 µM). Scale bar, 20 µm. (B) TUNEL assay. Apoptosis detection in MCF 7 cells treated with CMF, curcumin or citral and the combination of curcumin and citral for 24, 48, and 72 hours. Cells were observed under microscope and % apoptotic cells were counted out of total 100 cells. The image showed the stained cells treated with curcumin (40 µM) and citral (80 µM). Scale bar, 10 µm. The data represent the means of three independent experiments performed in triplicate, with standard deviations represented by vertical bars. *p< 0.05. |
 | Figure 5Measurement of DNA damage in MCF 7 cells caused by curcumin and citral treatment. (A) The DNA damage in MCF 7 cells exposed to CMF, curcumin or citral and the combination of curcumin and citral were assessed by COMET assay. A1, untreated MCF 10 A cells; A2, untreated MCF 7 cells; A3, CMF treated MCF 7 cells, and A4, curcumun (40 µM) and citral (80 µM) treated MCF 7 cells. (B) Graph showed the measure of COMET parameters. The data represent the means of three independent experiments performed in triplicate. |
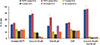 | Figure 6Distribution of MCF 7 cells in various phases of cell cycle; exposed to cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), curcumin or citral and the combination of curcumin and citral; analyzed by flow cytometry. The data represent the means of three independent experiments performed in triplicate, with standard deviations represented by vertical bars. *p<0.05. |
 | Figure 7Intracellular reactive oxygen species (ROS) generation and total glutathione (GSH) content. (A) Effect of curcumin and citral on intracellular ROS. The graph shows the generation of ROS in MCF7 and MCF 10A cells, treated with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), curcumin or citral and the combination of curcumin and citral. The data represent the means of three independent experiments performed in triplicate, with standard deviations represented by vertical bars. (B) Effect of curcumin and citral on the total GSH content. The total GSH content of MCF 7 cells exposed to cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), curcumin or citral and the combination of curcumin and citral was measured. Values in graph represent the total GSH content in µM. The data represent the means of three independent experiments performed in triplicate, with standard deviations represented by vertical bars. *p<0.05. |
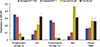 | Figure 8Measurement of levels of apoptotic proteins. The apoptotic protein levels in the MCF 7cells treated with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), curcumin or citral and the combination of curcumin and citral in MCF 7 and untreated MCF 10A cells were measured using the Sandwich Enzyme-Linked Immunosorbent Assay kit. Values of all protein represented their absorbance at 450 nm. The data represent the means of three independent experiments performed in triplicate, with standard deviations represented by vertical bars. *p<0.05. |
ACKNOWLEDGMENTS
The authors are grateful to the B. R. D. School of Biosciences, Sardar Patel University for providing basic facilities to carry out the present scientific work. The first author is also thankful to P. D. Patel Institute of Applied Sciences, CHARUSAT, for allowing to use the cell culture facilities; and Anand Agriculture University to provide instrumental facilities.
References
1. Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008; 37:2558–2574.

2. Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011; 11:144.

3. Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009; 11:495–510.

4. Ramachandran C, You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res Treat. 1999; 54:269–278.

5. Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann AS. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J Adv Pharm Technol Res. 2011; 2:3–8.

6. Rabbani SI, Devi K, Shivananda TN. Studies on antimutagenic effects of citral in mice. J Food Agric Environ. 2004; 2:62–64.
7. Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007; 96:2181–2196.

8. Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008; 104:657–667.

9. Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999; 456:311–314.

10. Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002; 62:3868–3875.
11. Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005; 71:484–488.

12. Xia H, Liang W, Song Q, Chen X, Chen X, Hong J. The in vitro study of apoptosis in NB4 cell induced by citral. Cytotechnology. 2013; 65:49–57.

13. Zhang T, Zhang Q, Chen D, Jiang J, Zhou Q. Growth inhibition of human breast cancer cell line MDA-MB-231 by rosiglitazone through activation of PPARgamma. Chin J Clin Oncol. 2008; 5:407–412.

14. Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005; 110:173–183.

15. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000; 35:206–221.

16. Sies H, Akerboom TP. Glutathione disulfide (GSSG) efflux from cells and tissues. Methods Enzymol. 1984; 105:445–451.
17. Singh RP, Dhanalakshmi S, Agarwal R. Phytochemicals as cell cycle modulators: a less toxic approach in halting human cancers. Cell Cycle. 2002; 1:156–161.

18. Chaouki W, Leger DY, Liagre B, Beneytout JL, Hmamouchi M. Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin Pharmacol. 2009; 23:549–556.

19. Shen L, Ji HF. Theoretical study on physicochemical properties of curcumin. Spectrochim Acta A Mol Biomol Spectrosc. 2007; 67:619–623.

20. Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009; 16:916–922.

21. Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004; 3:1101–1108.
22. Fang HY, Chen SB, Guo DJ, Pan SY, Yu ZL. Proteomic identification of differentially expressed proteins in curcumin-treated MCF-7 cells. Phytomedicine. 2011; 18:697–703.

23. Shyur LF, Lee SH, Chang ST, Lo CP, Kuo YH, Wang SY. Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways. Phytomedicine. 2010; 18:16–24.

24. Ataee R, Ataie A, Shadifar M, Nasri N, Hagghi H, Hayati E. Synergic effect of curcumin and melatonin on proliferation and apoptosis of HT29 colorectal cancer cell line. Res Pharm Sci. 2012; 7:S117.
25. Li H, Jin L, Wu F, Li X, You J, Cao Z, et al. Effect of curcumin on proliferation, cell cycle, and caspases and MCF-7 cells. Afr J Pharm Pharmacol. 2012; 6:864–870.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download