INTRODUCTION
Until 2002, patients with ipsilateral supraclavicular lymph node (ISCLN) metastasis were considered to have a poor prognosis and were only offered palliative treatment [
1]. However, Brito et al. [
2] reported that patients with ISCLN metastasis at diagnosis had a similar clinical course and prognosis to stage IIIB locally advanced breast cancer, when compared to patients with distant metastasis. On the basis of these data, the sixth edition of the American Joint Committee on Cancer-Tumor Node Metastasis (AJCC-TNM) staging system reclassified ISCLN metastasis from M1 to N3c [
3].
Only a limited number of retrospective studies have focused on the prognosis of patients with synchronous and/or metachronous ISCLN metastasis; however, these studies are important because prospective study of these patients is unattainable [
24567]. Nevertheless, they have several limitations including very small sample sizes, inhomogeneous study populations, selection bias, and absence of pathological proof for ISCLN metastasis, which results in overstaging. The latter limitation affects survival outcome and even occurred in the study by Brito et al. [
2] that included patients from prospective trials.
Ultrasonography is a commonly used procedure for detecting and staging lymph node metastases. However, the sensitivity and specificity of ultrasonography for lymph node metastasis is 66%-73% and 44%-98%, respectively [
8]. Therefore, although previous studies have reported the outcomes for N3c breast cancer, the prognosis could not be determined. Furthermore, three recent trials have shown the benefit of adjuvant taxanes, and taxane-based chemotherapy has become an important component of adjuvant treatment for breast cancer [
91011]. Yet these previous studies cannot assess the effects of era-specific therapies. Therefore, the prognosis of N3c breast cancer is still unclear, and the optimal locoregional treatment for the supraclavicular area is controversial.
We investigated the prognosis, patterns of failure, and prognostic factors for breast cancer patients with pathologically proven synchronous ISCLN metastases.
METHODS
Patient selection
We reviewed the records of breast cancer patients at a single institution who had pathological proof of ISCLN metastases at diagnosis, between 1990 and 2010. The ISCLN involvement of all patients was diagnosed by using ultrasound-guided fine-needle aspiration. The eligibility criteria were as follows: Eastern Cooperative Oncology Group performance status 0 or 1; no evidence of distant metastases; no other primary cancer; nonpregnant women; noninflammatory breast cancer; and unilateral breast cancer. This study was approved by the Institutional Review Board of the Asan Medical Center (2015-0210).
All patients were clinically staged according to the 2002 AJCC staging system for breast cancer [
3]. Patients were assessed at presentation using clinical history, physical examination, mammography, ultrasonography, chest computed tomography (CT), breast magnetic resonance imaging, bone scintigraphy, positron emission tomography-CT (PET/CT), and biopsy of the breast and suspicious lymph nodes.
Treatment
Surgery consisted of either mastectomy or breast-conserving surgery (BCS). BCS was carefully performed according to the preference of the patients and our institutional selection criteria; when there was a lack of initial extensive skin and chest wall involvement and absence of extensive microcalcifications and multifocal disease; potential achievement of clear surgical margins with adequate residual breast tissue after BCS; and no contraindication to radiotherapy (RT).
Chemotherapy was performed before or after surgery according to the physician's preference. Until 2002, the cyclophosphamide, methotrexate, and fluorouracil regimen or cyclophosphamide, doxorubicin, and fluorouracil regimen was used, while a taxane-based regimen has been used since 2003. Before 2009, neoadjuvant chemotherapy was administered for one or two cycles to decrease tumor size, but since 2009, it has been used to a greater extent. Hormone suppression therapy was administered to patients with estrogen receptor-positive or progesterone receptor-positive breast cancer.
RT was targeted to the breast or chest wall and regional lymphatics including the ipsilateral axillary apex and supraclavicular fossa compartment. Before 1995, two-dimensional simulation was used, and after 1995, CT simulation and three-dimensional conformal planning were performed. The radiation dose varied depending on the patient's and physician's preferences. In general, 50-50.4 Gy (median, 50.4 Gy) was delivered in 25-28 fractions (median, 28 fractions) to the breast or chest wall by using tangential fields. A 10-Gy boost to the tumor bed was performed in patients who had undergone BCS. The axillary apex and supraclavicular fossa were irradiated with 45-50.4 Gy (median, 50.4 Gy) by using a posterior axillary boost or an appositional field technique, followed by a 10-15 Gy boost in some patients who did not undergo ISCLN excision. For patients who had clinically detected internal mammary lymph node metastasis, 50-50.4 Gy was delivered to the internal mammary regions, with a 10-Gy boost.
Local aggressive treatment was defined as treatment including breast surgery, axillary lymph node dissection (ALND), ISCLN excision, RT, and chemotherapy. It was performed routinely for patients with ISCLN metastases at our institution. However, the following patient groups did not receive local aggressive treatment: those who were treated prior to 2002; those who experienced progression during neoadjuvant or adjuvant chemotherapy; and those who refused treatment.
Statistical analysis
Locoregional failure (LRF) was defined as recurrence in or progression to the ipsilateral breast or chest wall or the regional nodal station (ipsilateral axillary, supraclavicular, or internal mammary lymph nodes). Distant failure (DF) was defined as recurrence away from the locoregional site. Disease-free status was defined as no evidence of LRF, DF, or death. Locoregional failure-free survival (LRFFS), distant failure-free survival (DFFS), disease-free survival (DFS), and overall survival (OS) were calculated using the Kaplan-Meier method, and comparisons between the groups were performed by using the log-rank test. Multivariate analysis was performed by using the Cox proportional hazards model. All analyses were two-sided, and a p-value <0.05 was considered significant. All statistical analyses were performed by using the SPSS statistical package version 18.0 (SPSS Inc., Chicago, USA).
RESULTS
Patient characteristics
Of the patients enrolled in the registry, 111 met the selection criteria. The median age was 49 years (range, 27-81 years). Eighty patients (72.1%) had tumors ≤20 mm, and 31 patients (27.9%) had tumors >20 mm. Sixteen patients (14.4%) had clinically detected internal mammary lymph node metastasis. Hormone receptors (HRs) and human epidermal growth factor receptor 2 were positive in 53 (47.7%) and 48 (43.2%) patients, respectively. Seventy (63.1%) and 30 (27.0%) patients underwent mastectomy and BCS, respectively. Ninety-eight (88.3%) patients received ALND. RT was performed in 100 patients (90.1%), and the median radiation dose delivered to the supraclavicular fossa was 50.4 Gy (range, 45-66 Gy). The patient, disease, and treatment characteristics are summarized in
Table 1.
Survival outcomes, patterns of failure, and variables affecting survival
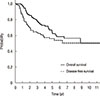
For the 111 patients, the 5-year OS and DFS rates were 64.2% and 56.2%, respectively (
Figure 1). During the follow-up period, 49 patients (44.1%) experienced recurrence. Of these, 17 (15.3%), 20 (18.0%), and 12 (10.8%) had LRF, DF, and LRF and DF as the first recurrence. On univariate analysis, RT, ALND, trastuzumab treatment, HR status, and local aggressive treatment were identified as significant factors for OS. Hormone therapy showed a trend for superior OS rate. RT, trastuzumab treatment, and ALND were significant factors affecting DFS, while type of breast surgery, HR status, and local aggressive treatment showed marginal significance for DFS. Multivariate analysis included only those variables with a
p-value <0.1, as determined in the univariate analysis. Multivariate analysis showed that RT, HR status, and trastuzumab were significant variables for OS and DFS. The univariate and multivariate analyses are summarized in
Tables 2 and
3, respectively.
Eighty-nine patients who received surgery, ALND, ISCLN biopsy only or excision, RT, and chemotherapy were analyzed. There was no significant difference in LRFFS, DFFS, or OS when ISCLN biopsy only and excision were compared. The 5-year LRFFS, DFFS, and OS rates for 16 patients who received surgery, ALND, ISCLN biopsy only, RT, and chemotherapy, were 66.0%, 61.1%, and 64.3%, respectively. The 5-year LRFFS, DFFS, and OS rates for 73 patients who received local aggressive treatment in the form of ISCLN excision, were 76.5%, 64.8%, and 70.9%, respectively.
DISCUSSION
Only a few retrospective studies have evaluated breast cancer patients with synchronous ISCLN metastasis [
24567]. Moreover, four of these studies did not have histologic proof of ISCLN metastasis, including the study by Brito et al. [
2], which analyzed patients from prospective trials, and one other study [
6] with pathological proof of ISCLN metastasis included a small number of patients. Therefore, the prognosis of N3c breast cancer is still ambiguous.
In the present study, we evaluated 111 patients with ISCLN metastases that were confirmed pathologically. Furthermore, our institution has performed PET/CT for patients with locally advanced breast cancer since 2005, and therefore, we were able to analyze a homogeneous cohort of N3c breast cancer patients. In our study, the 5-year OS rate (64.2%) was better than that of previous reports. Multivariate analysis showed that RT, HR status, and trastuzumab treatment were significant variables for OS. In our study, 104 (93.7%), 58 (52.3%), and 32 (28.8%) patients received chemotherapy, hormone therapy, and trastuzumab therapy, respectively. We assumed that more number of patients, who received taxane-based chemotherapy regimens, hormone therapy, and trastuzumab treatment, showed a better survival rate compared to that observed in previous studies (
Table 4). After the three recent trials that have shown a benefit for adjuvant taxanes, taxane-based chemotherapy has become an important component of adjuvant treatment for breast cancer [
91011]. However, the previous studies were performed before the benefit of adjuvant taxanes was known. Another possible reason for superior survival in the present study is that a high proportion of patients underwent PET/CT. As 82 patients (74%) were staged with PET/CT, we were able to exclude asymptomatic M1 patients. Park et al. [
7] also demonstrated a high 5-year OS rate (78%), with a relatively high proportion of patients receiving taxane-based chemotherapy, hormone therapy, and trastuzumab treatment. Moreover, as all the patients in that study underwent PET/CT, they were able to exclude those with metastatic disease also.
Seventy-three (65.8%) patients received local aggressive treatment that included surgery, ALND, ISCLN excision, RT, and chemotherapy. The 5-year OS rate of patients who received local aggressive treatment was significantly superior to that of patients who received nonaggressive treatment (70.9% vs. 49.3%,
p=0.036) (
Figure 2A). The LRFFS rate was higher in patients who underwent local aggressive treatment than in those who underwent nonaggressive treatment (
p=0.017) (
Figure 2B). However, there was no significant difference in DFFS when these two groups were compared (
p=0.368) (
Figure 2C). Furthermore, local aggressive treatment did not show a significant difference for OS on multivariate analysis, and whether aggressive treatment is a predictive or a prognostic value remains to be determined.
The role of ISCLN excision is uncertain; 85 patients (76.6%) underwent ISCLN excision, but it did not affect LRFFS, DFFS, or OS in this study. Eighty-nine patients received surgery, ALND, ISCLN biopsy or excision, RT, and chemotherapy. There was no difference in LRFFS, DFFS, or OS when ISCLN biopsy and excision were compared. Huang et al. [
5] reported that the surgical removal of supraclavicular disease after chemotherapy might only benefit patients with residual supraclavicular disease, which could be a source of tumor cell dissemination, and result in distant metastases and locoregional recurrence. The value of RT dose to the supraclavicular fossa is also unclear. In the present study, the RT dose to the supraclavicular fossa (≥50.4 Gy vs. <50.4 Gy) did not affect LRFFS (
p=0.427). As various treatments were performed based on the patients' response to neoadjuvant chemotherapy, it is difficult to deduce the effect of the RT dose on the supraclavicular fossa or ISCLN excision. Therefore, supraclavicular excision and the supraclavicular fossa boost routinely yield inconclusive results, and it remains to be determined which modality should be omitted or performed more aggressively.
The present study has limitations in terms of its retrospective design, including potential selection bias and variable treatment strategies depending on the time-period. Nevertheless, this study has unique strengths in that we utilized a large homogeneous study cohort from a single institution, with pathologic proof of ISCLN metastases, and we excluded patients with occult distant metastasis by using PET/CT. Further studies should be performed to investigate the optimal RT dose and value of supraclavicular excision.
In conclusion, multimodality treatment with surgery, chemotherapy, hormone therapy, and RT is strongly recommended for breast cancer patients with synchronous ISCLN metastases.









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download