Abstract
Purpose
Despite the fact that the androgen receptor (AR) is known to be involved in the pathogenesis of breast cancer, its prognostic effect remains controversial. In this meta-analysis, we explored AR expression and its impact on survival outcomes in breast cancer.
Methods
We searched PubMed, EMBASE, Cochrane Library, ScienceDirect, SpringerLink, and Ovid databases and references of articles to identify studies reporting data until December 2013. Disease-free survival (DFS) and overall survival (OS) were analyzed by extracting the number of patients with recurrence and survival according to AR expression.
Results
There were 16 articles that met the criteria for inclusion in our meta-analysis. DFS and OS were significantly longer in patients with AR expression compared with patients without AR expression (odds ratio [OR], 0.60; 95% confidence interval [CI], 0.40-0.90; OR, 0.53; 95% CI, 0.38-0.73, respectively). In addition, hormone receptor (HR) positive patients had a longer DFS when AR was also expressed (OR, 0.63; 95% CI, 0.41-0.98). For patients with triple negative breast cancer (TNBC), AR expression was also associated with longer DFS and OS (OR, 0.44, 95% CI, 0.26-0.75; OR, 0.26, 95% CI, 0.12-0.55, respectively). Furthermore, AR expression was associated with a longer DFS and OS in women (OR, 0.42, 95% CI, 0.27-0.64; OR, 0.47, 95% CI, 0.38-0.59, respectively). However, in men, AR expression was associated with a worse DFS (OR, 6.00; 95% CI, 1.46-24.73).
Breast cancer is the most common malignancy among women in the world and the third leading cause of death after lung and colorectal cancer in United States [12]. It is a very heterogeneous disease, which shows diverse therapeutic response and clinical outcomes according to hormonal receptor (HR) status, lymph node status, molecular subtype, cancer stem cell marker status, and other factors [345]. Traditionally, HR status, especially the estrogen receptor (ER) and progesterone receptor (PR), is a very well-known factor associated with clinical outcomes in breast cancer patients [67]. These two receptors are extremely important to establish therapeutic strategies, predict the prognosis, and assess the biology of the tumor [89].
Recently, the androgen receptor (AR), which is a member of the steroid superfamily, has emerged as a prognostic factor [1011]. This receptor has two cysteine zinc finger motifs and a C-terminal extension-rich DNA binding domain, which is pivotal for AR-specific DNA binding [11]. AR agonists, such as testosterone and 5-α dihydrotestosterone, induce dimerization through the interaction between the N-terminal and C-terminal [11]. The AR is stabilized through this process [11]. In addition, the AR could also be activated by growth factors instead of testosterone [12]. Furthermore, in women, adrenal and ovarian androgens are sources of pre- and postmenopausal estrogens by converting into estradiol-17 [12]. In this way, the AR is thought to be involved in the pathogenesis of breast cancer. However, the clinical importance of the AR is not well established, and its significance as an independent predictor of clinical outcome remains controversial.
Therefore, we performed a meta-analysis to compare the proportion of patients with disease-free survival (DFS) and overall survival (OS) between AR expression and no AR expression in breast cancer to assess its prognostic role.
To evaluate the impact of AR expression on breast cancer survival, we searched PubMed, EMBASE, Cochrane Library, ScienceDirect, SpringerLink, and Ovid databases from inception to December 2013. The searched keywords were "breast cancer AND androgen receptor." In addition, the authors checked every reference of the articles.
The investigators independently reviewed every searched article and determined whether the articles were suitable according the following inclusion criteria: original article, reports that showed the survival outcomes after primary surgery, studies without neoadjuvant anticancer therapy, research without preoperative metastasis of breast cancer at any site, histologically verified expression of AR, number of patients with DFS or OS was presented according to the expression of AR, and publication in English. Review articles, editorial letters, case reports, animal testing research papers, poster presentations, duplicated articles, and non-English articles were excluded. If there was disagreement about the selection of studies among investigators, this was discussed until a consensus was reached.
The endpoint of this study was to evaluate the effect of AR expression on DFS and OS outcomes. DFS was defined as the length of time between the primary surgery and recurrence. OS was defined as the length of time between the primary surgery and date of death.
Cochran's Q and I2 statistics were used to assess the heterogeneity of the included studies. Statistical heterogeneity was suggested when the p-value was less than 0.1 [13]. In addition, the I2 index was used to classify heterogeneity into three groups (<25%: low, 25%-75%: moderate, >75%: high) [14]. If heterogeneity existed, the DerSimonian and Laird random effects model was used to account for variation within studies. Additionally, the Mantel-Haenszel fixed effects model was applied when the p-value was more than 0.1. The odds ratio (OR) with 95% confidence interval (CI) was estimated to present the relative frequency of death or recurrence between AR positive and negative breast cancers. The authors performed sensitivity analysis to assess the impact that changes in a certain parameter would have on the results of this meta-analysis.
Every statistical analysis was done by using Review Manager 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). p-values less than 0.05 were considered statistically significant.
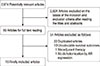
Figure 1 shows the flow chart of this meta-analysis. A total of 2,674 articles were found by using the keywords described above. Finally, 16 articles were selected and analyzed. Duplicated articles were judged based on the authors, titles, and contents. The characteristics of the studies included in the final analysis are shown in Table 1 [15161718192021222324252627282930].
To evaluate publication bias, we analyzed funnel plots of DFS and OS. The funnel plots showed symmetrical scatter plots, indicating no publication bias (Figure 2).
Figure 3 shows study heterogeneity and survival outcomes according to AR expression. A total of 12 and 14 articles reported data for DFS and OS, respectively. In the analysis of DFS, the included studies were highly heterogeneous (p<0.001, I2 index=76%), and in the analysis of OS, the studies showed moderate heterogeneity (p=0.002, I2 index=60%). In cases of tumors with AR expression, DFS was significantly longer compared with tumors without AR expression (OR, 0.60; 95% CI, 0.40-0.90; p=0.010). Similarly, OS was improved in tumors with AR expression compared without AR expression (OR, 0.53; 95% CI, 0.38-0.73; p<0.001).
The survival outcomes between tumors with AR expression and those without AR expression stratified according to HR expression are shown in Figure 4. We dichotomized the hormonal receptor status into HR positive (ER+ or PR+) and negative (ER- and PR-). A total of seven articles reported data for HR status. In patients with HR-positive tumors, those with AR expression had a significantly longer DFS (OR, 0.63; 95% CI, 0.41-0.98; p=0.040). In the analysis of OS, however, the data showed no significant differences (OR, 0.53; 95% CI, 0.23-1.24; p=0.140). Similarly, in cases of HR-negative tumors, DFS showed no significant survival differences regardless of AR expression (OR, 0.89; 95% CI, 0.39-2.03; p=0.790). However, in the analysis of OS, AR expression was associated with worse survival outcomes compared with patients without AR expression (OR, 1.43; 95% CI, 1.02-2.01; p=0.040).
Figure 5 shows the survival association between AR expression and molecular subtype. We dichotomized the molecular subtypes into triple-negative breast cancer (TNBC) and non-TNBC. Among all studies, articles were only included in this analysis if the article showed the status of all three receptors (i.e., ER, PR, and human epidermal growth factor receptor 2 [HER2]). A total of five studies reported data for molecular subtypes. With regard to TNBC, AR expression was associated with a significantly longer DFS and OS (OR, 0.44, 95% CI, 0.26-0.75, p=0.002; and OR, 0.26, 95% CI, 0.12-0.55, p<0.001, respectively). In contrast, there were no survival benefits of AR expression in patients with non-TNBC (OR, 0.75, 95% CI, 0.42-1.33, p=0.320; and OR, 0.72, 95% CI, 0.29-1.76, p=0.470 for DFS and OS, respectively).
The survival outcomes between tumors with AR expression and those without AR expression by gender are shown in Figure 6. Among all studies, articles were only included in this analysis when the gender of the patients was described or could be determined by description (such as, premenopausal, postmenopausal, etc.). Among 16 studies, we could identify the gender of the included patients in eight articles. Only one of eight research studies evaluated the survival outcomes in men. For women, AR expression was associated with longer DFS and OS (OR, 0.42, 95% CI, 0.27-0.64, p<0.001; and OR, 0.47, 95% CI, 0.38-0.59, p<0.001, respectively); in contrast, AR expression showed no statistical relation with OS in men (OR, 2.13; 95% CI, 0.55-8.14; p=0.270). Furthermore, AR expression in men was associated with a worse DFS than in patients without AR expression (OR, 6.00; 95% CI, 1.46-24.73; p=0.010).
Exclusion of the single article that analyzed men had no statistical effect on survival outcomes (OR, 0.53, 95% CI, 0.37-0.78; OR, 0.50, 95% CI, 0.37-0.69 for DFS and OS, respectively) according to AR expression. In the remaining 15 studies, AR expression was determined by using immunohistochemistry (IHC), radioimmunoassay (RIA), and reverse-phase protein lysate microarray (RPLM) in 12, two, and one article, respectively. Removal of the three studies in which AR expression was determined by using RIA and RPLM had no influence on the DFS and OS (OR, 0.52, 95% CI, 0.32-0.84; OR, 0.46, 95% CI, 0.31-0.68, respectively) according to AR expression. Two of the 12 remaining articles determined AR expression by using an IHC score and Allred score instead of the staining rate only. Removal of those two articles had no effect on DFS and OS (OR, 0.52, 95% CI, 0.29-0.93; OR, 0.46, 95% CI, 0.29-0.74, respectively). Among the remaining articles, one used a definition of AR expression that was too high. With the exception of that single article, there were no significant effects on survival outcomes (OR, 0.52, 95% CI, 0.29-0.93; OR, 0.47, 95% CI, 0.28-0.80 for DFS and OS, respectively). In addition, among the studies using IHC, the relationship between AR expression and OS showed no significant differences according to the cutoff value (p=0.435 among the cutoff values of 1%, 5%, and 10%). Thus, the different cutoff values (1%, 5%, and 10%) for determining AR expression had no effect on the survival outcomes. Furthermore, 11 articles researched DFS and OS regardless of molecular subtype. In these studies, AR expression had no significant effect on the survival outcomes (OR, 0.52, 95% CI, 0.31-0.88; OR, 0.49, 95% CI, 0.38-0.63 for DFS and OS, respectively).
In this study, we investigated survival outcomes of patients with breast cancer through the analysis of DFS and OS according to AR expression. We identified research studies that analyzed AR expression with a histological diagnosis after surgery. Ultimately, 16 studies that included 5,420 patients were enrolled in this meta-analysis.
As well known, meta-analyses have the potential for publication bias, because negative results are less likely to be published. However, we included studies that published negative results and confirmed that there was no publication bias by using funnel plots.
As mentioned above, the association between AR expression and survival outcomes remains controversial. Therefore, we tried to investigate the clinical significance of AR expression. In this study, we hypothesized that AR expression might be associated with the survival outcomes of patients with breast cancer. In addition, we analyzed the effect of AR expression on survival outcomes according to various parameters.
First, we estimated the association between AR expression and DFS and OS. In this meta-analysis, we verified the association between AR expression and improved survival outcomes. This result was consistent with our hypothesis.
Second, we investigated whether an association exists between AR expression and survival outcomes according to HR expression. The results showed that when the tumors expressed HR and AR, only DFS was significantly improved. In patients with no HR expression, however, there was no improvement in DFS. Furthermore, OS was longer in patients with no AR expression rather than in those with AR expression. Some possible reasons could be suggested to explain this result. At first, endocrine therapy could affect the function of AR as well as HR. Patients with HR-positive tumors might receive endocrine therapy, and this therapy might have an effect on AR. This cascade might lead to the prevention of breast cancer recurrence in patients with AR-expressing tumors. In addition, if there is no expression of HR, the androgenic pathway of breast cancer might be more enhanced by AR activation. Therefore, AR expression might result in a negative effect on survival outcome. However, as this is merely the present authors' hypothesis, more research will be needed.
Third, the authors analyzed the survival association between AR expression and molecular subtype. The results showed that when AR was expressed, survival outcomes were improved in patients with TNBC. However, there was no improvement in survival among non-TNBC patients. This result might indicate that the pathogenesis of breast cancer is different by molecular subtype. In non-TNBC, the three types of receptor (i.e., ER, PR, and HER2) might be more associated with the pathogenesis of breast cancer than the AR. In contrast, in TNBC, the AR might be more related to the pathogenesis of breast cancer than the three receptors. However, as this hypothesis has not been demonstrated experimentally, additional studies are required.
Last, we evaluated the association between AR expression and survival outcomes according to gender. In men AR expression was not significantly associated with OS. Moreover, AR expression in men was associated with a lower DFS. This result might be because of the different levels of sex hormone according to gender. However, as only one of the included articles researched the relationship between AR expression and survival outcomes in male patients, additional studies with larger cohorts are needed.
This study had some limitations. First, we were unable to analyze hazard ratios due to the programmatic limitation of Review Manager, which does not have the ability to calculate the value. In addition, because the authors extracted numerical survival data from some articles through graphs, texts, and tables, hazard ratios could not be analyzed. Therefore, ORs were the only way to analyze the survival data. However, because odds ratios do not include the concept of time, this could have resulted in bias. Second, there was moderate-to-high heterogeneity in the analysis of DFS and OS. This heterogeneity could have resulted from the differences in adjuvant therapy, number of patients, etc. Therefore, a survival analysis after correcting for contributing factors might be needed.
In conclusion, expression of the AR in breast cancer might be associated with improved survival outcomes, especially in patients with HR-positive tumors, TNBC, and women. On the basis of this meta-analysis, we suggest that the existence of AR-positive tumors is related with prognostic features and contributes to clinical outcomes.
Figures and Tables
 | Figure 2Funnel plots of (A) disease-free survival and (B) overall survival of the included studies. The funnel plots showed symmetricity which proposed no publication bias.SE=standard error; OR=odds ratio.
|
 | Figure 3Forest plots of (A) disease-free survival (DFS) and (B) overall survival (OS) of the included studies. In androgen receptor (AR) expressed tumors, DFS and OS showed statistically significant improvement compared with no AR expression.M-H=Mantel-Haenszel; CI=confidence interval. *5-Year survival data.
|
 | Figure 4Forest plots of survival outcomes according to hormonal receptor status. (A) Disease-free survival and (B) overall survival in patients with hormonal receptor expression. (C) Disease-free survival and (D) overall survival in patients without hormonal receptor expression.M-H=Mantel-Haenszel; CI=confidence interval; AR= androgen receptor. *5-Year survival data.
|
 | Figure 5Forest plots of survival outcomes according to molecular subtypes. (A) Disease-free survival and (B) overall survival in patients with triple-negative breast cancer (TNBC). (C) Disease-free survival and (D) overall survival in patients with non-TNBC.M-H=Mantel-Haenszel; CI=confidence interval; AR=androgen receptor.
|
 | Figure 6Forest plots of survival outcomes by gender. (A) Disease-free survival and (B) overall survival in men. (C) Disease-free survival and (D) overall survival in women.M-H=Mantel-Haenszel; CI=confidence interval; AR=androgen receptor. *5-Year survival data.
|
Table 1
Characteristics of the included studies

| Author | Country (yr) | No. of patients | Median age yr (range) | Gender | Median follow-up mo (range) | Subtype | AR assessment | Definition of AR+ |
|---|---|---|---|---|---|---|---|---|
| Agoff [15] | USA (2003) | 78 | 54.9* (26-91) | Women | 25* | ER (-) | IHC | > 5% staining of tumor cells |
| Luo [16] | China (2010) | 269 | 49 (25-80) | Women | - | TNBC | IHC | IHC score† ≥2 |
| Non-TNBC | ||||||||
| Micello [17] | Italy (2010) | 226 | 58.7* (24-97) | - | 116.4 (83-173) | ER and PR (-) and HER2 (+) | IHC | ≥ 10% nuclear staining |
| Peters [18] | USA (2009) | 215 | - | - | - | Not related‡ | IHC | ≥ 75% nuclear staining |
| Hu [19] | USA (2011) | 1,467 | 61 (30-55) | Women | 168 | Not related‡ | IHC | ≥ 1% nuclear staining |
| Kuenen-Boumeester [20] | The Netherlands (1996) | 153 | 55*(29-88) | Women | 46 (12-73) | Not related‡ | IHC | ≥ 0% staining of tumor cells |
| Yu [21] | China (2011) | 327 | 52.5* | - | 66 | Luminal A, B | IHC | Allred score§ ≥3 |
| HER2 (+) | ||||||||
| Basal like | ||||||||
| Normal like | ||||||||
| Castellano [22] | 3 Countries∥ (2010) | 859 | - | - | 82 | ER (+) | IHC | ≥ 1% staining of tumor cells |
| Peters [23] | Australia (2012) | 73 | 54*(30-94) | - | - | - | IHC | ≥ 10% nuclear staining |
| Agrawal [24] | Poland (2008) | 488 | 54.3* (32-79) | Women | - | Not related‡ | IHC | Not described |
| Collett [25] | Norway (1996) | 269 | - | Women | - | Not related‡ | RIA | ≥ 43 fmol/mg |
| Gonzalez-Angulo [26] | USA (2009) | 347 | 59 (23-89) | - | 50.4 (9.6-110.4) | TNBC | RPLM | Above median expression |
| ER and/or PR (+), HER2 (-) | ||||||||
| HER2 (+) | ||||||||
| He [28] | Poland (2003) | 43 | 65 (29-85) | Men | 48 (3-130) | Not related‡ | IHC | ≥ 10% staining of tumor cells |
| Kwiatkowska [27] | China (2011) | 287 | - | Women | 72 (8-182) | TNBC | IHC | ≥ 5% staining of tumor cells |
| Søreide [29] | Noway (1992) | 224 | 60.5 (21-89) | Women | - | Not related‡ | RIA | > 50.5 pmol/g protein |
| Qu [30] | China (2013) | 109 | 58 ± 13¶ | - | 74.4 | Not related‡ | IHC | > 1% nuclear staining |
AR=androgen receptor; ER=estrogen receptor; IHC=immunohistochemistry; TNBC=triple-negative breast cancer; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; RIA=radioimmunoassay; RPLM=reverse-phase protein lysate microarray.
*Mean value; †(score of staining intensity)×(score of percentage of positive cells); ‡Regardless of subtypes; §(score of staining intensity)+(score of percentage of positive cells); ∥Australia, Italy, and Switzerland; ¶Mean±SD.
References
2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–2150.

3. Tsutsui S, Kataoka A, Ohno S, Murakami S, Kinoshita J, Hachitanda Y. Prognostic and predictive value of epidermal growth factor receptor in recurrent breast cancer. Clin Cancer Res. 2002; 8:3454–3460.
4. Chang ED, Lee EJ, Oh SJ, Kim JS, Kang CS. Prognostic factors predicting the survival of breast cancer patients with metastases or recurrences: clinicopathologic characteristics of primary tumor. J Breast Cancer. 2005; 8:62–67.

5. Kim YS, Jung MJ, Ryu DW, Lee CH. Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer. 2014; 17:121–128.

6. Samaan NA, Buzdar AU, Aldinger KA, Schultz PN, Yang KP, Romsdahl MM, et al. Estrogen receptor: a prognostic factor in breast cancer. Cancer. 1981; 47:554–560.

7. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–2502.

8. Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008; 14:8019–8026.

9. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast cancer. Seventh international conference on adjuvant therapy of primary breast cancer. J Clin Oncol. 2001; 19:3817–3827.

10. Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010; 21:488–492.

11. Higa GM, Fell RG. Sex hormone receptor repertoire in breast cancer. Int J Breast Cancer. 2013; 2013:284036.

12. Bryś M. Androgens and androgen receptor: do they play a role in breast cancer? Med Sci Monit. 2000; 6:433–438.
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

15. Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003; 120:725–731.

16. Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer. 2010; 29:585–590.

17. Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010; 457:467–476.

18. Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009; 69:6131–6140.

19. Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011; 17:1867–1874.

20. Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, et al. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996; 32A:1560–1565.

21. Yu Q, Niu Y, Liu N, Zhang JZ, Liu TJ, Zhang RJ, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011; 22:1288–1294.

22. Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010; 124:607–617.

23. Peters KM, Edwards SL, Nair SS, French JD, Bailey PJ, Salkield K, et al. Androgen receptor expression predicts breast cancer survival: the role of genetic and epigenetic events. BMC Cancer. 2012; 12:132.

24. Agrawal AK, Jeleń M, Grzebieniak Z, Zukrowski P, Rudnicki J, Nienartowicz E. Androgen receptors as a prognostic and predictive factor in breast cancer. Folia Histochem Cytobiol. 2008; 46:269–276.

25. Collett K, Mæhle BO, Skjærven R, Aas T. Prognostic role of oestrogen, progesterone and androgen receptor in relation to patient age in patients with breast cancer. Breast. 1996; 5:123–126.

26. Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009; 15:2472–2478.

27. Kwiatkowska E, Teresiak M, Filas V, Karczewska A, Breborowicz D, Mackiewicz A. BRCA2 mutations and androgen receptor expression as independent predictors of outcome of male breast cancer patients. Clin Cancer Res. 2003; 9:4452–4459.
28. He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, et al. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2012; 29:406–410.

29. Søreide JA, Lea OA, Varhaug JE, Skarstein A, Kvinnsland S. Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol. 1992; 18:112–118.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download