Abstract
Whole-body bone scans and whole body 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic scans are sensitive for detecting bone metastasis in patients with breast cancer. However, it is often difficult to discriminate between bone metastasis and other nonmalignant bone lesions. Polyostotic fibrous dysplasia is a rare disorder characterized by the osteoid medullary cavity filling with fibrous tissue causing bony expansion. We report the case of a 42-year-old female patient with ductal carcinoma in situ, which appeared to have multiple bone metastases on initial work-up images. Subsequently, the bone metastases were identified as polyostotic fibrous dysplasia. The patient underwent modified radical mastectomy and subsequently visited for a second opinion regarding the bony metastases. She underwent right ilium computed tomography-guided biopsy. Pathology was consistent with fibrous dysplasia. This patient received only adjuvant tamoxifen, and 1.5 years later, there was no evidence of recurrence.
Polyostotic fibrous dysplasia is characterized by the medullary cavity of bones becoming filled with fibrous tissue, and its etiology remains unknown [1,2]. Clinically, it is important to distinguish fibrous dysplasia from distant bone metastasis. Polyostotic fibrous dysplasia may closely mimic the appearance of bony metastatic disease on radiological examinations [3]. Only one case report has distinguished breast cancer bone metastasis from polyostotic fibrous dysplasia according to whole-body 18F-fluorodeoxyglucose (18F-FDG) positron emission tomographic/computed tomographic (PET/CT) scan [4].
We report our experience with a case of polyostotic fibrous dysplasia that mimicked breast cancer bone metastasis.
A 42-year-old woman was admitted to the breast clinic of a university hospital with a palpable mass in the right breast originally detected in a local clinic. According to her medical records, there were no signs of nipple retraction or skin abnormalities. Breast ultrasonography showed a 1.2-cm microlobulated heterogeneously hypoechoic mass in the lower inner quadrant of the right breast. A core needle biopsy was performed, and ductal carcinoma in situ (DCIS) was diagnosed. The patient had no past medical or family history of breast disease and had no bone pain symptoms.
She underwent chest radiography as a baseline study for general anesthesia, which showed osteolytic lesions of the right second, third, eighth ribs, and the left fourth rib (Figure 1). A chest CT was performed and revealed multiple asymmetric osteolytic lesions in the previously enumerated ribs suggesting multiple bone metastases. She was indicated for a whole-body bone scan to identify distant metastases. The scan revealed lesions of the right parietal and occipital bones, cervical, thoracic, lumbar, sacral (CTLS) spine, bilateral scapulae, multiple ribs, bilateral pelvic bones, right femur, tibia, fibula, right tarsal bone, sternum, and right humerus (Figure 2). 18F-FDG PET/CT revealed an increased uptake in the right femur, right ninth and left fourth ribs, and T-L spine consistent with multiple bone metastases. Serum laboratory test results and tumor markers were not increased as follows: calcium, 8.9 mg/dL; phosphorus, 3.2 mg/dL; alkaline phosphatase, 85 IU/L; carcinoembryonic antigen, 0.807 ng/mL; carbohydrate antigen 15-3, 3.87 U/mL.
The patient's medical history included a modified radical mastectomy of the right breast out of concern for a possible hidden malignancy. Histopathological examination showed a 2.0×1.6-cm DCIS with nuclear grade 2, and no metastatic lymph nodes detected out of the 24 dissected axillary lymph nodes. Immunohistochemistry studies showed positive staining for estrogen receptor protein and progesterone receptor protein, and negative staining for human epidermal growth factor receptor 2 (score 1). The patient underwent a postoperative pelvic bone biopsy given the histopathological stage, which was nondiagnostic.
The patient visited another hospital for a second opinion, and we conducted a multidisciplinary review. The final histopathological report by the pathologist confirmed the previous diagnosis of DCIS (Figure 3). Radiological evaluation demonstrated the aforementioned results and was not able to exclude the possibility of multiple bone metastases. We recommended a repeat bone biopsy based on the knowledge that distant metastasis after a diagnosis of pure DCIS is rare. The patient underwent right ilium CT-guided biopsy, which revealed fibro-osseous lesions consistent with fibrous dysplasia (Figure 4). She only received tamoxifen (20 mg daily) endocrine therapy. At 1-year follow-up, a whole-body bone scan was performed (Figure 5). At 1.5 years, a 18F-FDG PET/CT scan revealed only persistent increased uptake (Figure 6) without evidence of recurrence.
The National Comprehensive Cancer Network (NCCN) guidelines (ver. 3.2013) state that routine systemic staging is not indicated for early breast cancer in the absence of symptoms. Bone scans are indicated only in the presence of localized bone pain or elevated alkaline phosphatase, and 18F-FDG PET/CT can be considered for clinical stage IIIA malignancy or higher. The initial work-up for stage 0, DCIS, includes taking a history, physical examination, bilateral mammograms, evaluation of estrogen receptor status, pathological review, and genetic counseling if there is high risk for hereditary breast cancer, and optional breast magnetic resonance imaging [5].
Plain radiography may not reveal a metastatic bone lesion until extensive marrow replacement has occurred. Bone scans and 18F-FDG PET/CT scans are recognized as more sensitive for the detection bone metastasis. Bone scanning is a sensitive test for the detection of metastatic breast cancer, but not all abnormal findings are diagnostic of skeletal metastasis. Some studies have found a relatively low rate (≤5%) of abnormal scans in patients with stage I and II breast cancers and only half of those with positive scans subsequently had documented bony metastasis [6].
Moon et al. [7] found that whole-body PET imaging had high diagnostic accuracy for patients with suspected recurrent or metastatic breast carcinoma. Choi et al. [8] found that the sensitivity and specificity of 18F-FDG PET/CT in detecting distant metastasis were 100% and 96.4%, respectively, whereas those of conventional imaging were 61.5% and 99.2%, respectively. Cook et al. [9] found that 18F-FDG PET/CT was superior to bone scanning for detecting osteolytic breast cancer metastases. However, 18F-FDG PET/CT detected fewer bone metastases compared with 99mTc-methylene diphosphonate bone scintigraphy in a subgroup of patients with osteoblastic disease.
Nuclear medicine imaging plays an important role in the diagnosis of benign and malignant bone lesions. Whole-body bone scanning is widely available and commonly used to detect metastases from malignant tumors, is more sensitive than radiography and allows the whole body to be surveyed [3]. However, many benign osteolytic lesions such as eosinophilic granulomas, multiple myeloma, disseminated tuberculosis, fibrous dysplasia, and enchondroma appear very similar to bone metastasis on bone scans [3,10]. These limitations on the diagnosis of bone metastasis by bone scans and 18F-FDG PET/CT emphasize the importance of clinical and radiological correlation, and most cases will benefit from pathological confirmation. Image-guided percutaneous bone biopsy has a high diagnostic yield and accuracy. Studies of percutaneous CT-guided bone biopsy combined with fine-needle aspiration report a positive predictive value of 82% and a negative predictive value of 100% [11].
Fibrous dysplasia is a benign tumor in which masses of fibroblasts and islands of cartilage replace bone marrow in one or more locations. It is commonly found in young patients with mild bone pain or as an incidental finding, although the lesions may persist into late adulthood. The main value of whole-body bone scans in fibrous dysplasia is to confirm polyostotic involvement; however, sometimes these lesions may show only minimal uptake or even no uptake [12]. Fibrous dysplasia presents in three forms: monostotic, polyostotic, and polyostotic with endocrine diseases. The monostotic form of fibrous dysplasia accounts for 80% to 85% of cases [13]. Three percent of patients with the polyostotic form have endocrine disease and are cases of McCune-Albright syndrome. McCune-Albright syndrome is a sporadic disorder characterized by the triad of irregularly edged hyperpigmented macules (café au lait spots), a slowly progressive bone disorder usually involving the base of the skull and long bones, such as polyostotic fibrous dysplasia and luteinizing hormone-releasing hormone-independent precocious puberty [2]. This patient had no other symptoms of McCune-Albright syndrome. Polyostotic fibrous dysplasia is very hard to differentiate from disseminated bone metastasis. Even when combined with a patient's medical history and multiple imaging techniques, the clinical diagnosis of metastatic lesions remains a challenge. The clinical characteristics of polyostotic fibrous dysplasia and malignant bone metastasis in multiple sites are very similar, consequently differentiating between the two is important when determining the appropriate therapy. Some case reports have described breast cancer occurring in patients with McCune-Albright syndrome. However, patients with breast cancer and polyostotic fibrous dysplasia have rarely been reported, and these reports only addressed the difficulty of differentiating bone metastasis from fibrous dysplasia by radiological techniques [14].
Distant metastasis after a diagnosis of pure DCIS is rare (0.14%), but bone is the most common distant metastatic site in DCIS patients [15]. The diagnosis of bone metastasis in patients with early breast cancer is very important because it has a significant impact on treatment planning. If bone metastasis is suspected, based on radiological imaging, we recommend further imaging and confirmation by pathology to rule out alternative bone diseases.
This case highlights the potential pitfalls in the interpretation of bone scans and 18F-FDG PET/CT when widespread abnormal areas of increased uptake are seen, particularly in the context of suspected malignancy. Polyostotic fibrous dysplasia may closely mimic the appearance of bony metastatic disease. In these instances, clinical and radiological correlation is helpful in determining the etiology, and pathological confirmation is recommended.
Figures and Tables
Figure 1
The chest X-ray as a baseline study for general anesthesia. Osteolytic lesions of the right second, third, eighth, and left fourth ribs, which is suggestive of multiple bone metastases.
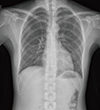
Figure 2
The baseline whole body bone scan. Abnormal increased uptake was demonstrated in the right parietal, occipital, cervical, thoracic, lumbar, sacral spine, bilateral scapulae, multiple ribs, bilateral pelvic bones, right femur, tibia, fibula, right tarsal bone, sternum, and right humerus.
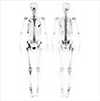
Figure 3
Microscopic examination of breast tumor. Cribriform-tumor clusters, with architectural and nuclear atypia, consistent with ductal carcinoma in situ (A, H&E stain, ×40; B, H&E stain, ×200).
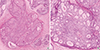
Figure 4
Microscopic examination of biopsied specimen of the right ilium. Photomicrograph was demonstrated irregularly shaped islands of woven bone with a bland spindle cell background stroma (H&E stain, ×200).

Figure 5
Follow-up bone scan after 1 year. Increased radio-uptake in the skull, right second, third, fourth, sixth, eighth, and left fourth ribs, right ilium, right ischium, right acetabulum, right femur, right tibia, right fibula, and second phalanx of right foot, which is suggestive of polyostotic fibrous dysplasia, but which cannot exclude combined metastasis.

Figure 6
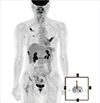
Follow-up 18F-fluorodeoxyglucose (FDG) positron emission tomographic/computed tomographic scan after 1.5 years. Increased FDG uptake in the right first, second, third, eighth, and left fourth ribs, right femur, right ischium, and right ilium, which is suggestive of polyostotic fibrous dysplasia, but which cannot exclude multiple metastases.
Notes
References
1. MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009; 38:196–215.

2. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991; 325:1688–1695.

3. Zhang Y, Zhao C, Liu H, Hou H, Zhang H. Multiple metastasis-like bone lesions in scintigraphic imaging. J Biomed Biotechnol. 2012; 2012:957364.

4. Shigesawa T, Sugawara Y, Shinohara I, Fujii T, Mochizuki T, Morishige I. Bone metastasis detected by FDG PET in a patient with breast cancer and fibrous dysplasia. Clin Nucl Med. 2005; 30:571–573.

5. Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, et al. Breast cancer, version 3. 2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013; 11:753–760.
6. Lee YT. Bone scanning in patients with early breast carcinoma: should it be a routine staging procedure? Cancer. 1981; 47:486–495.

7. Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998; 39:431–435.
8. Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, et al. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer. 2012; 15:441–448.

9. Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998; 16:3375–3379.

10. Wang K, Allen L, Fung E, Chan CC, Chan JC, Griffith JF. Bone scintigraphy in common tumors with osteolytic components. Clin Nucl Med. 2005; 30:655–671.

11. Leffler SG, Chew FS. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic yield and accuracy. AJR Am J Roentgenol. 1999; 172:1389–1392.

12. Han J, Ryu JS, Shin MJ, Kang GH, Lee HK. Fibrous dysplasia with barely increased uptake on bone scan: a case report. Clin Nucl Med. 2000; 25:785–788.
13. Lustig LR, Holliday MJ, McCarthy EF, Nager GT. Fibrous dysplasia involving the skull base and temporal bone. Arch Otolaryngol Head Neck Surg. 2001; 127:1239–1247.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download