Abstract
Purpose
The objectives of this study were to assess the potential value of Ki-67 in predicting response to neoadjuvant chemotherapy in breast cancer patients and to suggest a reasonable cutoff value for classifying Ki-67 expression.
Methods
This study included 74 breast cancer patients who underwent surgery after anthracycline-based neoadjuvant chemotherapy between 2007 and 2012. We analyzed the clinical and immunohistochemical characteristics using core biopsy specimens obtained before neoadjuvant chemotherapy to determine their correlations with the response to chemotherapy.
Results
A clinical complete response was observed in 6 patients (8.1%); a clinical partial response, in 44 patients (59.5%); and clinical stable disease, in 24 patients (32.4%). A pathologic complete response (pCR) was observed in 10 patients (13.5%). In univariate analysis, estrogen receptor (ER) negativity (p=0.031), human epidermal growth factor receptor 2 (HER2) positivity (p=0.040), and high Ki-67 expression (p=0.036) were predictive factors for a pCR. In multivariate analysis, Ki-67 was the only independent predictor of a pCR (p=0.049). The analysis of Ki-67 values revealed that 25% was a reasonable cutoff value for predicting the response to chemotherapy. In subgroup analysis, a higher Ki-67 value (≥25%) was a significant predictive factor for the response to neoadjuvant chemotherapy, especially in ER-negative and HER2-positive breast cancer patients.
Conclusion
Ki-67 expression in breast cancer tissue may be an effective factor for predicting the response to neoadjuvant chemotherapy. We suggest that a 25% level of Ki-67 expression is a reasonable cutoff value for predicting a response to chemotherapy. Moreover, Ki-67 is a useful predictive factor for pCR, especially in patients with ER-negative and HER2-positive breast cancer.
Ki-67 is a nuclear antigen that is expressed in the growth and synthesis phases of the cell cycle, but not in the resting phase [1]. Since its discovery in the early 1980s, Ki-67 has received a lot of attention as a proliferation marker in almost all types of cancers. Numerous studies have demonstrated that the expression of the human Ki-67 protein is associated with cell proliferation; therefore, increased Ki-67 levels in cancer tissue have been considered a poor prognostic factor, while also being a positive predictive factor for response to chemotherapy [2,3].
However, the clinical role of Ki-67 has not been established; therefore, the routine use of Ki-67 as a predictor of prognosis and treatment response in breast cancer patients is not recommended [4]. Data that support the routine use of Ki-67 are currently insufficient, and the cutoff values to define high versus low Ki-67 expression are vague and inaccurate.
In many cases, neoadjuvant chemotherapy reduces tumor size, which enables patients who were initially inoperable to undergo mastectomy and makes breast-conserving surgery possible in patients who otherwise would have required mastectomy [5]. The outcome of neoadjuvant chemotherapy can be determined in a relatively short time, which makes this approach useful for deciding which drugs or regimens are effective for specific pathologic conditions. Moreover, this is a useful modality for investigating the efficacy of specific biologic markers as predictive and prognostic factors. Neoadjuvant chemotherapy does not provide a survival advantage compared to postoperative adjuvant therapy [6]. However, patients who achieve a pathologic complete response (pCR) have significantly improved disease-free survival and overall survival compared to those with residual cancer [7].
The objectives of this study were to assess the potential value of Ki-67 in predicting the therapeutic response to neoadjuvant chemotherapy in breast cancer patients and to determine the best Ki-67 cutoff value that increases the prediction accuracy in making treatment decisions. We also correlated Ki-67 expression with the chemosensitivity of tumors in different breast cancer subgroups to determine the subgroups in which Ki-67 is most valuable as a predictive biomarker.
The 74 patients included in this retrospective study were selected from the breast cancer patients who underwent radical surgery after neoadjuvant chemotherapy at Gachon University Gil Hospital between January 2007 and December 2012. All patients were pathologically diagnosed with invasive breast cancer via core needle biopsy. At diagnosis, all patients had clinical stage II or stage III tumors. To exclude the possibility of distant metastasis, the patients underwent a staging workup, including chest computed tomography, abdominal computed tomography, and bone scintigraphy. The clinical and pathologic characteristics of all patients were obtained from the hospital medical records. Patient characteristics were available, which included the age, menopausal status, clinical stage, systemic chemotherapy regimen, clinical response, type of surgery, pathologic stage, histological grade, hormone receptor status, human epidermal growth factor receptor 2 (HER2) status, p53 expression, and Ki-67 expression level. The Institutional Review Board of Gachon University Gil Hospital approved this study (GCIRB2013-116).
All patients enrolled in this study received anthracycline-based neoadjuvant chemotherapy. In our hospital, the common regimens for neoadjuvant chemotherapy were six cycles of AT (50 mg/m2 doxorubicin and 75 mg/m2 docetaxel every 3 weeks), four cycles of CEF (500 mg/m2 cyclophosphamide, 100 mg/m2 epirubicin, and 500 mg/m2 fluorouracil on days 1 and 8 every 3 weeks), and four cycles of CAF (500 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, and 500 mg/m2 fluorouracil every 3 weeks). Surgery was performed 3 to 4 weeks after the final dose of neoadjuvant chemotherapy. We decided whether to perform breast-conserving surgery or mastectomy after considering the ability to remove residual disease completely using optimal cosmetic results. Axillary lymph node dissection was performed in all patients. Targeted therapy with trastuzumab was administered to all patients with HER2-positive tumor cells.
Pathologic specimens of the tumors of all patients were available. Specialists in breast pathology at the Department of Pathology, Gachon University Gil Hospital, evaluated all the pathologic specimens. Pathologists analyzed the samples obtained via core needle biopsy before initiating chemotherapy, as well as the samples obtained via surgical resection. The histological type, histological grade, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, p53 expression and Ki-67 expression level of the specimens obtained via both biopsy and surgery were evaluated. We used the pathologic and immunohistochemical findings of core needle biopsy specimens to analyze the predictive factors associated with a response to neoadjuvant chemotherapy.
The primary antibodies used for each immunohistochemical staining were the monoclonal anti-ER protein (ID5, 1:50 dilution; Dako, Glostrup, Denmark), monoclonal anti-PR protein (PgR636, 1:100 dilution; Dako), monoclonal anti-HER2 protein (CB11, 1:100 dilution; NeoMarker, Fremont, USA), monoclonal anti-p53 protein (DO07, 1:100 dilution; Novocastra, Newcastle, UK), and monoclonal anti-KI-67 protein (MIB-1, 1:400 dilution; Dako). After washing the tissue sections with the buffer, they were sequentially incubated with biotinylated secondary antibody (1:500 dilution; Dako) and Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, USA) diluted in phosphate-buffered saline for 30 minutes at room temperature. Normal breast tissue was used as a negative control in each staining batch.
The ER and PR statuses were determined using the Allred scoring system, and a positive result was defined as a total score of ≥3. The HER2 status was determined using the HercepTest (Dako), and 3+ reactions were considered to be a positive results, whereas 0 and 1+ reactions were considered negative. If 2+ results were obtained using the Hercep Test scoring system, fluorescence in situ hybridization was performed to determine the HER2 status. p53 expression was considered positive when staining was observed in more than 10% of tumor cells. The Ki-67 expression levels were expressed as the percentage of cells with positive nuclear staining among the total number of tumor cells (at least 1,000 were counted).
Prior to the start of chemotherapy, we measured the size of the primary breast tumor and recorded the axillary lymph node status of all patients using ultrasonography, magnetic resonance imaging, and computed tomography in order to determine the primary clinical stage. After neoadjuvant chemotherapy and before surgical resection, we measured the tumor size and axillary lymph node status again using ultrasonography and magnetic resonance imaging in order to evaluate the clinical response to chemotherapy.
The clinical response to chemotherapy was determined by comparing the baseline tumor size to the tumor size after neoadjuvant chemotherapy using radiological imaging. For the quantitative analysis of clinical response to chemotherapy, we used the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1 as follows: clinical complete response (cCR), disappearance of all target lesions with a reduction in the short axis of any pathologic lymph nodes to <10 mm; clinical partial response (cPR), a decrease of at least 30% in the sum of the diameters of the target lesions, with reference to the sum of the baseline diameters; clinical progressive disease (cPD), an increase of at least 20% in the sum of the diameters of the target lesions, with reference to the smallest sum recorded in the study; and clinical stable disease (cSD), the presence of lesions with neither sufficient shrinkage to qualify as cPR nor sufficient increase to qualify as cPD [8].
The pathologic response to chemotherapy was determined by analyzing the surgical tumor specimens. The residual tumor size and lymph node status were evaluated to assess the pathologic response to neoadjuvant chemotherapy. pCR was defined as no pathologic evidence of a residual invasive carcinoma in the breast or axillary lymph nodes. Residual ductal carcinoma in situ was included under pCR.
To determine the predictive factors for a clinical response after chemotherapy, we compared the baseline characteristics of patients with cCR, cPR, and cSD. The Kruskal-Wallis test was used to compare quantitative characteristics, and the Pearson chi-square test was used to compare categorical characteristics. To identify predictive factors for pCR, we compared the baseline characteristics of patients with pCR to those of patients without pCR. The Student t-test was used to compare quantitative characteristics, and the Pearson chi-square test was used to compare categorical characteristics. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive cutoff value for Ki-67 expression. To identify the predictive factors associated with pCR after neoadjuvant chemotherapy, multiple logistic regression analysis was performed. Statistical significance was calculated at the 95% confidence interval (p<0.05) and all analyses were performed using the SPSS version 18.0 for Windows (SPSS Inc., Chicago, USA).
Between January 2007 and December 2012, 74 patients (mean age, 44.7±9.2 years) underwent surgery after neoadjuvant chemotherapy for primary breast cancer. All patients had clinical stage II or stage III breast cancer and received anthracycline-based neoadjuvant chemotherapy. After neoadjuvant chemotherapy, six patients (8.1%) showed a cCR, 44 patients (59.5%) showed a cPR, and 24 patients (32.4%) showed cSD. Based on the RECIST criteria, there were no patients with cPD in this study. After neoadjuvant chemotherapy, 10 patients (13.5%) showed a pCR based on the analysis of surgical specimens. Patient characteristics and responses to neoadjuvant chemotherapy are shown in Tables 1 and 2.
We used ROC curve analysis to calculate the optimal cutoff value to classify Ki-67 expression for predicting a response to neoadjuvant chemotherapy (Figure 1). The area under the ROC curve (AUC) for Ki-67 expression was 0.774 (p<0.001; 95% confidence interval, 0.662-0.886), indicating that the baseline Ki-67 level based on a core biopsy specimen is a very useful predictive marker for response to neoadjuvant chemotherapy (Figure 1). We also observed that other variables had a relatively lower significance as predictors of response to neoadjuvant chemotherapy. The AUC for ER status was 0.523 (p=0.746), the AUC for HER2 status was 0.634 (p=0.063), and the AUC for tumor size was 0.641 (p=0.051) (Figure 1). Using ROC curve analysis, we identified 25% as the best cutoff level for Ki-67 expression in predicting a response to neoadjuvant chemotherapy. This cutoff value was associated with an optimal sensitivity of 71.0% and a specificity of 77.1% (Figure 1). For this cutoff value, the positive predictive value of Ki-67 was 85.7% and the negative predictive value was 56.3%.
In the univariate analysis of clinical response, a high Ki-67 expression level was the only significant factor for predicting better clinical response to neoadjuvant chemotherapy (p<0.001) (Table 1). In the univariate analysis of pathologic response, the probability of a pCR was significantly higher in breast cancer patients with an ER-negative status (p=0.031), a HER2-positive status (p=0.040), and a higher Ki-67 expression level (p=0.036) (Table 2). Multiple logistic regression analysis was performed using age, clinical tumor size, ER status, PR status, HER2 status, p53 expression and Ki-67 expression level (Table 3). We found that only the Ki-67 expression level was statistically significant as an independent predictor of a pCR (p=0.049).
We validated the ability of the 25% Ki-67 cutoff level to predict a pCR in response to neoadjuvant chemotherapy for different molecular subgroups of breast cancer (Table 4). A pCR was observed in two of the 15 patients (13.3%) in the ER-positive and HER2-positive subgroup, and only among patients with high Ki-67 levels. In the ER-positive and HER2-negative subgroup, only one patient (3.1%) achieved pCR, and she had high Ki-67 levels, as well. One patient (14.2%) in the ER-negative and HER2-negative subgroup achieved pCR, who also had high Ki-67 levels. The highest pCR rate (30.0%) was observed in the ER-negative and HER2-positive subgroup. Furthermore, the positive predictive value of Ki-67 levels increased up to 41.7% in this subgroup. The 25% Ki-67 expression cutoff value was useful for predicting pCR, especially in the ER-negative and HER2-positive subgroup (p=0.019).
A number of clinical trials have been undertaken to determine the predictive factors for clinical and pathologic responses to neoadjuvant chemotherapy, because prediction of chemotherapy outcomes helps doctors decide on the need for neoadjuvant chemotherapy. Since most chemotherapy drugs are associated with severe adverse effects, it is important to avoid unnecessary systemic chemotherapy that delays the administration of other treatments.
It is reasonable to presume that tumors with a higher level of cell proliferation might respond better to chemotherapy than tumors with lower proliferation. Ki-67 is widely known as a proliferative marker, and numerous studies have shown a positive correlation between Ki-67 expression and the proliferative cell fraction in tumors. Therefore, many clinicians have examined the relationship between Ki-67 expression and chemotherapy response in patients with breast cancer, and breast cancer with a high Ki-67 expression level has repeatedly been shown to respond better to chemotherapy [3,9]. However, there is no clear standard value for classifying Ki-67 expression as high or low, therefore, each study has used a different standard for the classification of Ki-67 levels. In a published paper on the prognostic factors for breast cancer, the researchers classified patients into two groups using a cutoff value of 12% for Ki-67 expression, with the reasoning that 12% was the median Ki-67 expression value of patients without recurrence [10]. Another related study adopted 20% as the cutoff value to distinguish between low and high Ki-67 expression, without any valid explanation [11]. Some studies have suggested 25% as the cutoff value, the same value as our study, to classify low versus high Ki-67 expression, with the reasoning that 25% was the median value of Ki-67 expression for the cohort of patients [12]. Although all the above studies proved that high Ki-67 expression was a predictive factor for pCR, such arbitrary criteria can be adjusted to result in a desired positive outcome. It is important to establish a standard system of measurement and rational criteria for the cutoff value for Ki-67 expression, and we suggested a methodology to determine a reasonable cutoff value for Ki-67 expression, in the hope that the results of this study will be validated in multicenter studies and will contribute to the establishment of rational criteria for the determination of Ki-67 expression.
Calculation of the Ki-67 cutoff value is one of the most meaningful aspects of the present study. ROC curve analysis was adopted to determine the cutoff value for the classification of Ki-67 expression in predicting the response to neoadjuvant chemotherapy. There is always a compromise between sensitivity and specificity, since a higher sensitivity will be accompanied by a lower specificity and vice versa. We decided that a 25% Ki-67 level was the optimal cutoff point because it was the value with the largest sum of sensitivity and specificity. To our knowledge, this is the first time a study has suggested a quantified standard level for the classification of Ki-67 expression, which is relatively correct in its ability to predict the response to neoadjuvant chemotherapy in breast cancer. To validate the cutoff Ki-67 level calculated in this study, we examined its ability to predict pCR in patients with different molecular subgroups of breast cancer. We confirmed that the 25% cutoff point was a reliable value for predicting the response to neoadjuvant chemotherapy in breast cancer patients, especially in the subgroup with ER-negative and HER2-positive tumors. We also proved that Ki-67 is a superior predictor of response to chemotherapy by comparing the ROC curve of the Ki-67 expression level, tumor size, ER status, PR status, and HER2 status.
In our study, the ER and HER2 status correlated with pCR, similar to the results of a previously published study [13]. The Ki-67 level also strongly correlated with pCR and clinical response according to RESIST, version 1.1. In multivariate analysis, only the Ki-67 expression level was a statistically significant independent predictor of pCR. With respect to the molecular subtypes of breast cancer, as defined by the ER status and HER2 status of tumors, the pCR rates in patients with ER-positive and HER2-positive (13.3%), ER-positive and HER2-negative (3.1%), ER-negative and HER2-positive (30.0%), and ER-negative and HER2-negative (14.2%) breast cancer were also consistent with the results of previously published studies [13,14]. The Ki-67 expression level seemed to be an especially valuable predictor in patients with ER-negative and HER2-positive breast cancer, such that the positive predictive value for pCR increased up to 41.7% in this subgroup. These results suggest that Ki-67 evaluation is suitable for inclusion in routine pathologic examination for breast cancer, especially in cases where neoadjuvant chemotherapy is being considered.
The measurement of the Ki-67 level is a widely used method to assess tumor proliferation. Healthy breast tissue expresses low levels of Ki-67, below 3% [15]. The level of Ki-67 expression in breast cancer can be either a positive predictive factor for response to chemotherapy or a poor prognostic factor [9,16]. Owing to these paradoxical characteristics, it is difficult to declare whether Ki-67 is a prognostic marker of the final outcome. However, the pCR is regarded as a better prognostic factor for survival, and the significance of the Ki-67 expression level as a predictor of pCR cannot be denied.
The features of breast cancer vary according to the molecular subtype, enabling clinicians to administer tailored therapy for each breast cancer patient. In our study, the Ki-67 expression level had better predictive power for pCR in patients with ER-negative and HER2-positive breast cancer. The Ki-67 level can be used differently according to the molecular subtype of breast cancer. We recommend that the Ki-67 expression level be carefully analyzed in patients with ER-negative and HER2-positive breast cancer who are planning to receive neoadjuvant chemotherapy.
Ki-67 provides additional and independent predictive information regarding the response to neoadjuvant chemotherapy for breast cancer. Ki-67 expression should be carefully analyzed as a routine biological marker in breast cancer patients, especially in those who are candidates for neoadjuvant chemotherapy. Additionally, clinicians should more favorably consider neoadjuvant chemotherapy in cases of patients with high Ki-67, ER-negative, and HER2-positive breast cancer.
We propose that 25% might be used as a standard cutoff value for Ki-67 expression for predicting the response to neoadjuvant chemotherapy in breast cancer patients. Validation of this cutoff value in a larger population would be desirable, allowing adjustments for an ideal standard value for predicting pCR in response to neoadjuvant chemotherapy in breast cancer patients.
Figures and Tables
Figure 1
The comparison of predictive value for the response to neoadjuvant chemotherapy in breast cancer patients by receiver operating characteristic (ROC) curve analysis. The 25% Ki-67 cutoff point had the largest sum of sensitivity (71.0%) and specificity (77.1%).
T=tumor size; ER=estrogen receptor; HER2=human epidermal growth factor receptor 2; AUC=area under the ROC curve.

Table 1
Characteristics of patients according to clinical response to neoadjuvant chemotherapy
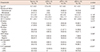
cCR=clinical complete response; cPR=clinical partial response; cSD=clinical stable disease; CEA=carcinoembryonic antigen; CA=carbohydrate antigen; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD; †p-value calculated by Kruskal-Wallis test; ‡p-value calculated by chi-square test.
Table 2
Characteristics of the patients according to pathologic response to neoadjuvant chemotherapy

References
1. Seidal T, Edvardsson H. Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology. 1999; 34:416–424.

2. de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007; 96:1504–1513.

3. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005; 23:7212–7220.

4. Jones RL, Smith IE. Neoadjuvant treatment for early-stage breast cancer: opportunities to assess tumour response. Lancet Oncol. 2006; 7:869–874.

5. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007; 25:5287–5312.

6. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.

7. Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005; 23:9304–9311.

8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.

9. Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011; 11:486.

10. Nishimura R, Osako T, Okumura Y, Hayashi M, Arima N. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast Cancer. 2010; 17:269–275.

11. Li XR, Liu M, Zhang YJ, Wang JD, Zheng YQ, Li J, et al. Evaluation of ER, PgR, HER-2, Ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol. 2011; 28:Suppl 1. S31–S38.

12. Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004; 10:6622–6628.

13. Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012; 15:203–210.

14. Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010; 17:2411–2418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download