Abstract
Purpose
A growing body of evidence indicates that zoledronic acid (ZA) can improve the clinical outcome in patients with breast cancer and low estrogen levels. In the present study, we aimed to investigate the survival benefit of ZA administration in postmenopausal Korean women with breast cancer who were also receiving aromatase inhibitors.
Methods
Between January 2004 and December 2010, 235 postmenopausal breast cancer patients undergoing aromatase inhibitor therapy were investigated. All patients were postmenopausal, as confirmed by laboratory tests. Of these patients, 77 received adjuvant upfront ZA for at least 1 year in addition to conventional adjuvant treatment. The remaining 158 patients never received ZA and were treated according to the St. Gallen guidelines.
Results
The baseline characteristics for ZA treatment were not different between the two groups. The median follow-up time was 62 months, and the patients who received ZA in addition to aromatase inhibitors showed a better recurrence-free survival compared to those who received aromatase inhibitors alone (p=0.035). On multivariate analysis, the patients who received ZA showed a better recurrence-free survival independent of the tumor size, nodal status, progesterone receptor, and histological grade. For this model, Harrell c index was 0.743. The hazard ratio of ZA use for recurrence-free survival was 0.12 (95% confidence interval, 0.01-0.99).
Zoledronic acid (ZA), a potent nitrogen-containing bisphosphonate, has been used in daily practice for the prevention of skeletal-related events that are caused by bone metastasis from diverse tumors [1,2,3]. Furthermore, recent clinical trials provided solid evidence that ZA effectively inhibits the loss of bone minerals in postmenopausal women with breast cancer or premenopausal women with chemotherapy-induced amenorrhea [4,5,6].
In addition, since many preclinical studies have demonstrated the antitumor effect of ZA in preventing or palliating metastatic tumors [7,8,9,10], clinical data on ZA use in breast cancer have been evaluated. Moreover, studies showing the clinical benefits of ZA therapy in patients with bone metastases have been reported [1,11]. Although the routine use of ZA as an adjuvant treatment in unselected breast cancer patients is still controversial, a growing body of evidence has shown that combined ZA and endocrine therapy can improve clinical outcomes in patients with breast cancer and low estrogen levels, who are also menopausal or receiving ovarian suppression [5,12,13].
On the basis of the above findings, the use of ZA as an additional adjuvant treatment has become increasingly widespread. Previously, we showed that ZA can prevent bone mineral loss, which is accelerated by aromatase inhibitors in postmenopausal Korean women with breast cancer [14]. In the present study, we aimed to assess the survival benefit of combined ZA and upfront aromatase inhibitor therapy in postmenopausal Korean women with breast cancer.
We identified 252 hormone receptor-positive women who received upfront adjuvant aromatase inhibitors after surgery between January 2004 and December 2010 at a single institute. Of the 252 patients, 17 were excluded because ZA was administered for less than a year. Finally, this study enrolled 235 postmenopausal women. Postmenopausal status was defined as the absence of menstruation for at least 12 months before surgery, and serum follicle stimulating hormone levels greater than 30 mIU/mL. Patients who underwent bilateral salpingo-oophorectomy prior to the diagnosis of breast cancer were not excluded. All patients received anastrozole (1 mg), letrozole (2.5 mg), or exemestane (25 mg) daily. Ten patients switched aromatase inhibitors due to drug intolerance. In total, 158 patients received the aromatase inhibitors alone and 77 patients received aromatase inhibitors in combination with ZA. ZA treatment was administered more than twice for at least 1 year. In such cases, 4 mg of ZA was administered intravenously every 3 to 6 months. None of these patients received oral bisphosphonates. All the patients were treated with or without chemotherapy according to the St. Gallen guidelines.
For the immunohistochemical evaluation, formalin-fixed, paraffin-embedded tissue sections from surgical specimens were stained with antibodies for the estrogen receptor (ER; Novocastra, Newcastle upon Tyne, UK), the progesterone receptor (PR; Novocastra), human epidermal growth factor receptor 2 (HER2; Ventana Medical Systems, Tucson, USA), and Ki-67 (Dako, Glostrup, Denmark). ER and PR statuses were determined by nuclear staining, which was graded from 0 to 8 using the Allred score [15]. The results were categorized as positive when the total score, expressed as the sum of the proportion score and intensity score, was 3 or more. For HER2 evaluation, membranous staining was graded as follows: score 0, 1+, 2+, and 3+ [16]. HER2 status was deemed to be positive with a score of 3+ and negative with a score of 0 or 1+. Tumors with a score of 2+ underwent fluorescence in situ hybridization using the PathVysion HER2 DNA Probe Kit (Abbott-Vysis, Des Plaines, USA).
The tumors were classified according to the American Joint Committee on Cancer staging system, seventh edition. The modified Scarf-Bloom-Richardson grading system was used for tumor grading. Adjuvant systemic therapy and/or radiotherapy were considered according to standard guidelines based on patient age, primary tumor characteristics, and axillary lymph node status. All patients received aromatase inhibitors as adjuvant endocrine treatment. The Institutional Review Board of Gangnam Severance Hospital, Korea, approved the study (Local Institutional Review Board number: 3-2014-0917) in accordance with good clinical practice guidelines and the Declaration of Helsinki.
Age is presented in this study as a median value with a range, and compared using the Mann-Whitney U-test. Discrete variables were compared by the chi-square test. The primary endpoint was recurrence-free survival (RFS). RFS was measured from the date of the first curative surgery to the date of the first locoregional recurrence or distant metastasis. The Kaplan-Meier method was utilized to estimate RFS. Metastasis-free survival (MFS) was calculated to the date of the first distant metastasis. Estimated survival curves were compared using the log-rank test. Significant prognostic factors associated with RFS were selected using Harrell c statistic [17], and a Cox proportional hazards regression model was applied for multivariate survival analysis. The SPSS version 18 (SPSS Inc., Chicago, USA) and R softwares (http://www.r-projet.org) were used to perform these analyses. Statistical significance was defined by a p-value <0.05.
The median age of 235 postmenopausal women was 56 years (range, 43-91 years). Natural menopause occurred in 228 women (97.0%), and seven women underwent bilateral salpingo-oophorectomy prior to breast cancer diagnosis. All patients began upfront aromatase inhibitor therapy as adjuvant endocrine treatment after breast surgery or completion of adjuvant chemotherapy. In total, 77 patients received ZA in addition to aromatase inhibitors, while 158 women only received aromatase inhibitors. The median duration of ZA infusions was 45 months (range, 13-69 months), while the median number of ZA infusions was 11 (range, 2-21). None of the ZA-treated patients had osteonecrosis of the jaw.
Demographics, clinicopathological characteristics, and adjuvant treatment between the two groups were similar (Table 1). In addition, there was no difference in the use of aromatase inhibitors between the groups (Table 2). Ten women switched to other aromatase inhibitors because of adverse effects. One patient from the ZA-untreated group received upfront exemestane for 5 years. The regimens of neoadjuvant/adjuvant chemotherapy did not differ between the two groups. Detailed information regarding adjuvant or neoadjuvant chemotherapy is provided in Supplementary Table 1.
The median follow-up time was 62 months, when 14 women had tumor recurrences. At the time of the first relapse, three women had locoregional recurrences and 11 had distant metastases. Of the three women with locoregional recurrences at first relapse, two patients had distant metastases. During the follow-up period, three deaths occurred.
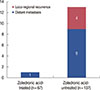
For all the patients, the 5-year RFS rate was 95.8%. The 5-year RFS rates for patients with and without ZA were 98.5% and 94.6%, respectively. The 5-year RFS rate for ZA-treated patients was significantly higher than that for ZA-untreated patients (p=0.022) (Figure 1A). Concurrently, MFS was significantly better for ZA-treated women than for ZA-untreated women (p=0.029) (Figure 1B). Among ZA-treated women, one case of distant metastasis was observed. In contrast, among the ZA-untreated women, three had locoregional recurrences and 10 had distant metastases at first relapse (Figure 2). Additionally, two of the three women with locoregional recurrences in the ZA-untreated group experienced distant metastasis.
In the univariate analysis of RFS, lower histological grade (p=0.012) and PR positivity (p=0.019) were associated with a better outcome (Supplementary Table 2). Multivariate analysis using a Cox proportional hazards regression model suggested that the use of ZA (adjusted hazard ratio, 0.12; 95% confidence interval, 0.02-0.99) was an independent prognostic factor for RFS, whereas PR positivity (adjusted hazard ratio, 0.29; 95% confidence interval, 0.09-0.98) was also associated with a decreased risk of tumor recurrence (Table 3). These differences were independent of tumor size, lymph node involvement, and histological grade. For this model, the Harrell c index was 0.743.
In Korea, the use of ZA for the preservation of bone mineral density in postmenopausal women treated with adjuvant aromatase inhibitors has been limited. We previously showed that the use of ZA can preserve bone mineral density in postmenopausal women treated with aromatase inhibitors [14]. In the present study, we provide evidence for the clinical benefits of adjuvant ZA therapy in postmenopausal women receiving aromatase inhibitors. Our data also demonstrate the antitumor effect of ZA in the inhibition of distant metastasis in postmenopausal breast cancer patients. One of the reasons why our data supports the clinical benefits of ZA is that the majority of the study population (63.0%) had true menopausal status. The median age of our study population was 56 years and 57% of the patients had experienced menopause more than 4 years ago.
Our finding that RFS was improved by the combined use of ZA and aromatase inhibitors in postmenopausal women is consistent with previous reports. Although the AZURE trial [12] failed to demonstrate the survival benefit of routine adjuvant ZA treatment among postmenopausal patients, the 5-year invasive disease-free survival rates differed significantly according to ZA treatment (78.2% in the ZA group and 71.0% in the control group). In the Austrian Breast Cancer Study Group Trial-12 (ABCSG-12) [13], the addition of ZA to endocrine therapy resulted in an absolute reduction of 3.2% points and a relative reduction of 36% in the risk of disease progression, as compared to endocrine therapy without ZA. In addition, the Zometa-Femara Adjuvant Synergy Trial (ZO-FAST) [5] showed that ZA administration in postmenopausal women receiving letrozole is associated with improved disease-free survival compared to those receiving letrozole alone. Taken together, these trials suggest that ZA in combination with endocrine therapy induces a consistent improvement of survival outcomes in women with low estrogen levels and is in concordance with our results.
To evaluate the direct antitumor effect of ZA in the clinical setting, several studies have attempted to test ZA as a component of neoadjuvant therapy. On the basis of short-term changes in biomarkers, several studies have provided evidence of the direct antitumor effect of ZA and the possible molecular mechanism for the biological effect of ZA [18,19]. Among the trials based on additional ZA treatment for neoadjuvant therapy, the FemZone trial [20] reported a trend suggesting a better response for letrozole in combination with ZA than letrozole alone. Coleman et al. [21] reported that addition of ZA to chemotherapy enhances the clinical response. Conversely, the neoadjuvant chemotherapy (TAC) with or without ZA (NEOZOTAC) trial [22] suggested that the addition of ZA to neoadjuvant chemotherapy did not improve the pathological or clinical response to chemotherapy. For postmenopausal women in the NEOZOTAC trial, the benefit of treatment with ZA was not statistically significant. Nevertheless, larger studies are warranted for the evaluation of the antitumor effect of ZA when it is administered as a component of neoadjuvant therapy in breast cancer patients, particularly in women with estrogen-poor environment.
Inherent limitations are associated with the retrospective nature of this study. Our study was not a randomized controlled study, and thus, selection bias may have affected patient classification for ZA treatment. In addition, uncontrolled adjuvant treatment can attenuate our findings. Heterogeneous regimens of neoadjuvant/adjuvant chemotherapy might potentially influence the survival outcome. A small sample size and very good outcomes in our population were other limitations. Although osteonecrosis of the jaw was not identified in ZA-treated patients, adverse effects of ZA administration were not systematically reviewed. Moreover, the duration of ZA infusions varied among the ZA-treated patients. Despite these limitations, our findings add to the growing body of data in support of adding ZA to aromatase inhibitor therapy in postmenopausal women. In addition, there was no difference in baseline characteristics and adjuvant treatment based on ZA treatment.
In conclusion, our findings suggest that upfront use of ZA might offer a survival benefit for postmenopausal patients receiving aromatase inhibitors.
Figures and Tables
 | Figure 1Kaplan-Meier plots according to zoledronic acid treatment. The p-value was calculated using log-rank test. (A) Recurrence-free survival (p=0.035). (B) Metastasis-free survival (p=0.037). |
References
2. Gnant M. Role of bisphosphonates in postmenopausal women with breast cancer. Cancer Treat Rev. 2014; 40:476–484.

3. Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003; 9:2394–2399.
4. Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008; 112:1001–1010.

5. Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013; 24:398–405.

6. Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007; 25:820–828.

7. Neville-Webbe HL, Coleman RE. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur J Cancer. 2010; 46:1211–1222.

8. Ottewell PD, Deux B, Mönkkönen H, Cross S, Coleman RE, Clezardin P, et al. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clin Cancer Res. 2008; 14:4658–4666.

9. Ottewell PD, Mönkkönen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008; 100:1167–1178.

10. Jeong J, Lee KS, Choi YK, Oh YJ, Lee HD. Preventive effects of zoledronic acid on bone metastasis in mice injected with human breast cancer cells. J Korean Med Sci. 2011; 26:1569–1575.

11. Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008; 113:193–201.

12. Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011; 365:1396–1405.

13. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009; 360:679–691.

14. Lee SA, Hwang SH, Ahn SG, Lee HM, Jeong J, Lee HD. Effects of zoledronic acid on bone mineral density during aromatase inhibitor treatment of Korean postmenopausal breast cancer patients. Breast Cancer Res Treat. 2011; 130:863–870.

15. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.
16. Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007; 25:5418–5425.

17. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.

18. Foroni C, Milan M, Strina C, Cappelletti M, Fumarola C, Bonelli M, et al. Pure anti-tumor effect of zoledronic acid in naive bone-only metastatic and locally advanced breast cancer: proof from the "biological window therapy". Breast Cancer Res Treat. 2014; 144:113–121.

19. Winter MC, Wilson C, Syddall SP, Cross SS, Evans A, Ingram CE, et al. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer: a randomized biomarker pilot study. Clin Cancer Res. 2013; 19:2755–2765.

20. Fasching PA, Jud SM, Hauschild M, Kümmel S, Schütte M, Warm M, et al. FemZone trial: a randomized phase II trial comparing neoadjuvant letrozole and zoledronic acid with letrozole in primary breast cancer patients. BMC Cancer. 2014; 14:66.

21. Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 2010; 102:1099–1105.

22. Charehbili A, van de Ven S, Smit VT, Meershoek-Klein Kranenbarg E, Hamdy NA, Putter H, et al. Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010-01). Ann Oncol. 2014; 25:998–1004.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download