Abstract
Purpose
DNA deacetylation by histone deacetylase (HDAC) is an important mechanism involved in the oncogenic tumorigenesis of breast cancer. Previous studies have reported an association of the estrogen receptor (ER) with HDACs and demonstrated the efficacy of HDAC inhibitors for the treatment of breast cancers via in vitro experiments. In this study, we examined the association of HDAC expression with clinicopathological parameters and disease-specific survival.
Methods
Immunohistochemical (IHC) analysis of HDAC1, HDAC2, HDAC3, and HDAC6 was performed using tissue microarrays in 300 invasive ductal carcinomas. IHC scoring was determined by multiplication of the intensity (0 to 3) and the proportion (0 to 4) of staining, and we classified tumors into low- and high-HDAC expression groups.
Results
High expression of HDAC1 was correlated with the molecular subtype (p=0.001) and human epidermal growth factor 2 (HER2) amplification (p=0.012). High expression of HDAC6 was correlated with a younger age (p<0.001), ER expression (p=0.025), progesterone receptor expression (p=0.034), molecular subtype (p=0.023), and HER2 amplification (p=0.011). High HDAC1 expression was correlated with luminal A tumors (p=0.001), while high HDAC6 expression was more common in luminal B tumors (p=0.023). Although the expression of HDACs did not exhibit prognostic significance in the entire cohort, high expression of HDAC1 and HDAC6 was associated with improved overall survival (OS) in patients with ER-positive tumors (p=0.017 and p=0.029, respectively), and high expression of HDAC2 was correlated with improved OS in ER-negative tumors (p=0.048) on univariate analysis. Furthermore, high HDAC6 expression was associated with improved disease-free survival (p=0.048) on multivariate analysis.
Conclusion
HDAC1 expression is significantly correlated with the molecular subtypes of tumors, with the highest expression being observed in luminal A tumors. HDAC6 is a significantly correlated with ER expression and the molecular subtype, thereby supporting the estrogen regulatory property of HDAC6. HDAC1 and HDAC6 expression are good prognostic factors for ER-positive tumors.
The role of epigenetic alterations, including the acetylation status of histones and DNA methylation, has been an important focus in studies on the development of human cancers, and such changes are often an early event in tumorigenesis [1,2]. Histone deacetylase (HDAC) family members remove acetyl groups from the lysine residues of histones, increasing ionic interactions between histones and DNA, and resulting in the formation of an inactive chromatin structure that represses DNA transcription [3]. Therefore, increased deacetylation of histones leads to cell proliferation, apoptosis, cell migration, and invasion via inactivation of tumor suppressor genes [4]. Recent studies using HDAC inhibitors (HDACIs) have demonstrated the in vivo and in vitro activities of HDACs affecting the cell cycle, apoptosis, and the differentiation of various cancers [5], and one of these HDACIs, suberoylanilide hydroxamic acid (SAHA), has been approved for the treatment of cutaneous T-cell lymphoma [6].
A total of 18 human HDAC isoenzymes have been described to date, and they are categorized into four classes: class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) are related to the yeast RPD3 deacetylase; class II HDACs are categorized into class IIa (HDAC4, HDAC5, HDAC7, and HDAC9) and class IIb (HDAC6 and HDAC10) and are homologous to the yeast Hda1 deacetylase; and class III HDACs include seven HDACs (SIRT1 to SIRT7), which show homology with the yeast Sir2 family [7]. Among these categories, class I and II HDACs are expressed at high levels in some cancers and appear to be involved in their carcinogenesis [2,8].
In breast cancers, HDACs have been highlighted due to several in vivo and in vitro studies demonstrating the increased activity of HDACs and the antitumor activity of HDACIs [9]. The most well known mechanism of action of HDACs involves their interaction with hormonal receptors (HR). Recent studies have revealed that the transcription of estrogen receptors (ERs) is regulated by epigenetic modifications, and they have described the efficacy of HDACIs through the re-expression of ERs [1]. Furthermore, the efficacy of HDACIs in treating human epidermal growth factor 2 (HER2)-amplified breast cancers has been reported in several in vitro studies [10,11].
Several studies have focused on class I HDACs, especially HDAC1, HDAC2, and HDAC3, and an ER-dependent class IIb HDAC, HDAC6, investigating their roles in breast carcinogenesis as well as their prognostic significances [2,9,12,13]. In this study, we analyzed the expression of HDAC1, HDAC2, HDAC3, and HDAC6 through immunohistochemical (IHC) analysis using a tissue microarray. We also analyzed the correlation with clinicopathological parameters and the prognostic significance of HDACs.
A total of 300 histologically proven invasive ductal carcinoma patients who underwent curative surgery between January 2003 and December 2008 at Hallym Sacred Heart Hospital were included in this study. Patients exhibiting pT4 disease or stage IV disease and those lacking pathology results were excluded from the study. Clinicopathological parameters, including tumor size, nodal status, margin status, and HER2 status were retrieved from pathology reports. HER2 status was interpreted according to the American Society of Clinical Oncology/College of American Pathologists guideline recommendations [14]. Other pathological parameters, including the histologic grade (HG), lymphatic tumor invasion, Ki-67 labeling index, and HR status, were evaluated after reviewing whole slides. HR statuses were evaluated according to the Allred score (Harvey) and a tumor was interpreted positive when the total score was >2. Breast cancer molecular subtypes were classified according to IHC profiles as described previously by Cheang et al. [15]. We obtained survival data from the breast cancer database of our institution and the Korean National Cancer Center database. This study was approved by the Institutional Ethics Committee of Hallym Sacred Heart Hospital (2014-I043).
IHC staining was performed on paraffin-embedded tissue sections using an automated IHC stainer (Ventana BenchMark TX; Ventana Medical System Inc., Tucson, USA) and iVIEW diaminobenzidine detection kits (Ventana Medical System Inc.), as previously described [16]. The following antibodies were used: monoclonal mouse anti-HDAC1 (dilution, 1:4,000; Abnova, Taipei, Taiwan); polyclonal rabbit anti-HDAC2 (dilution, 1:2,000; Abnova); polyclonal rabbit anti-HDAC3 (dilution, 1:100; Proteintech, Chicago, USA); monoclonal rabbit anti-HDAC6 (dilution, 1:200; Cell Signaling Technology, Beverly, USA); and monoclonal mouse p53 (dilution, 1:500; Novocastra, New Castle, UK). Briefly, IHC staining was performed as follows: 4-µm thick tissue sections were deparaffinized using EZ Prep solution (Ventana Medical System Inc.). A CC1 standard (pH 8.4 buffer containing Tris/borate/ethylenediaminetetraacetic acid) was applied for antigen retrieval at 99℃ for 60 minutes. The iVIEW inhibitor was blocked at 37℃ for 4 minutes. The slides were incubated with the primary antibodies at 42℃ for 32 minutes, followed by a secondary antibody against iVEW biotinylated Ig at 37℃ for 8 minutes. The slides were subsequently incubated in iVIEW streptavidin HRP at 37℃ for 8 minutes, followed by diaminobenzidine plus the H2O2 substrate for 8 minutes and then counterstained with hematoxylin and bluing reagent at 37℃. The reaction buffer (pH 7.6 Tris buffer) was used as the wash solution.
The IHC staining associated with HDACs was interpreted based on the intensity (0, negative; 1, mild; 2, moderate; 3, strong) and the proportion of positive cells (0, negative; 1, <10%; 2, ≥10% and <33%; 3, ≥33% and <66%; 4, ≥66%). The HDAC IHC scores were calculated through multiplication of the intensity and the proportion as described previously [2]. We classified the examined cases into two groups according to their IHC scores as follows: low expression (0-6) or high expression (8-12). The IHC staining of p53 was interpreted as positive when more than 10% of the tumor cells showed nuclear staining for p53.
IBM SPSS version 21 (IBM Inc., Armonk, USA) was used for statistical analysis. The chi-square test, Spearman correlation coefficient, and Fisher exact test were used for correlation analysis. Survival analysis was performed using the Kaplan-Meier method and a log-rank test for univariate analysis, and the Cox-proportional hazard models method for multivariate analysis. p-values of 0.05 or less were considered statistically significant.
The clinicopathological characteristics of the 300 cases are summarized in Table 1. The median age of the patients at the time of diagnosis was 51 years (range, 25-86 years), and the median follow-up duration was 70 months (range, 1-120 months). Most of the tumors were classified as pT1 (180 cases, 60.0%) or pT2 (114 cases, 38.0%), and the remaining six cases were classified as pT3. Younger patients (≤50 years) showed a higher incidence of both ER (67.1% vs. 53.5%, p=0.018) and progesterone receptor (PR) expression (70.6% vs. 51.6%, p=0.001). HER2 amplification was also observed more frequently in younger patients (32.2% vs. 19.7%, p=0.017). Tumors with a triple-negative phenotype were more common in older patients (28.0% vs. 11.9%, p=0.001).
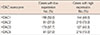
HDAC1 and HDAC2 were expressed in the nuclei of both normal and malignant epithelial cells. HDAC3 was observed in both the cytoplasm and the nuclei, and HDAC6 was observed in the cytoplasm (Figure 1). All of the HDACs were expressed in both tumor cells and normal epithelia, and the mean IHC scores for HDAC1, HDAC2, HDAC3, and HDAC6 among all cases were 6.17, 8.62, 6.26, and 8.17, respectively. High expression of HDAC1, HDAC2, HDAC3, and HDAC6 was observed in 144 (48.0%), 219 (73.0%), 127 (42.3%), and 219 (73.0%) cases, respectively (Table 2). The expression levels of all of the HDACs showed close correlations with each other (Spearman correlation coefficient, p<0.001). The p53 protein was expressed in the nuclei of tumor cells, and 163 cases (54.3%) were reactive for p53 protein.
HDAC1 expression was higher in tumors without HER2 amplification than in HER2-amplified tumors (52.5% vs. 35.1%, p=0.012). Although there was no correlation between HR and HDAC1, high HDAC1 expression was observed in luminal A tumors (p=0.001). High expression of HDAC6 was correlated with a younger age (p<0.001), ER-positive tumors (77.8% vs. 65.8%, p=0.025), PR-positive tumors (77.5% vs. 66.1%, p=0.034), and tumors showing HER2 amplification (84.4% vs. 69.1%, p=0.011) and expression of p53 (77.9% vs. 67.2%, p=0.038) (Table 3). There was no significant correlation between the expression of HDAC2 or HDAC3 and any of the available clinicopathological parameters.
Among the examined clinicopathological parameters, traditional prognostic factors including pT status (p=0.009), nodal status (p<0.001), molecular subtype (p=0.048), and the presence of lymphatic invasion (p=0.025) showed statistically significant associations with overall survival (OS) on univariate analysis. Univariate analysis also revealed that pT status (p=0.021), nodal status (p<0.001), molecular subtype (p=0.029), the presence of lymphatic invasion (p=0.016), ER expression (p=0.008), and PR expression (p=0.011) were significantly associated with disease-free survival (DFS). None of the HDACs showed a significant correlation with either OS (HDAC1, p=0.386; HDAC2, p=0.381; HDAC3, p=0.361; HDAC6, p=0.091) or DFS (HDAC1, p=0.747; HDAC2, p=0.596; HDAC3, p=0.383; HDAC6, p=0.194) on univariate analysis. Multivariate analysis incorporating pT status, nodal status, and molecular subtype was performed for the entire cohort. Nodal status and luminal B subtype were associated with poor OS, and nodal status and a triple-negative subtype showed a statistically significant association with poor DFS (Table 4). Although the prognostic significances of HDACs were not observed in the entire cohort, prognostic impacts of HDAC1 and HDAC6 expression were observed in certain subgroups of patients; in patients with ER-positive tumors, high HDAC1 expression predicted a significantly improved OS (p=0.017) (Figure 2A), and high HDAC6 expression also resulted in improved OS (p=0.029) (Figure 2C) on univariate analysis. However, high HDAC1 expression was not associated with improved DFS (p=0.421), but high HDAC6 expression was associated with improved DFS (p=0.021) (Figure 2E). Multivariate analysis in ER-positive tumors revealed that none of the parameters showed a statistically significant association with OS, but a high HG (p=0.023) and low HDAC6 expression (p=0.027) predicted a poor DFS (Table 5). Furthermore, high expression of HDAC6 was strongly associated with a better OS and DFS in luminal B tumors (p=0.001) (Figure 2D, F). High expression of HDAC2 was correlated with a significantly improved OS in patients with ER-negative tumors on univariate analysis (p=0.048) (Figure 2B), but HDAC2 expression was not associated with DFS (p=0.201) on univariate analysis. Multivariate analysis incorporating pT stage, nodal status, and HDAC expression also revealed a poor OS in the low HDAC2 expression group (hazard ratio, 2.715; 95% confidence interval, 0.999-7.380; p=0.050).
We demonstrated differential expression of HDAC1, HDAC2, HDAC3, and HDAC6 via IHC in invasive ductal carcinomas of the breast. The expression of HDAC1 was significantly associated with HER2 amplification, and high expression of HDAC6 was significantly correlated with a younger age, HR status, HER2 amplification, and p53 expression.
The association of HDAC activity with estrogen expression has been investigated in several studies. HDAC1 interacts with ER-α in vitro and in vivo and suppresses ER-α transcription through interaction with the activation function 2 domain of HDAC1 and the DNA-binding domain of ER-α [1]. HDAC6 is also an estrogen-regulated protein [13] and is related to cell migration and the transport of misfolded proteins via the deacetylation of tubulin [17,18]. Although the above in vitro studies reported an association of HDAC activities with ER expression, the following IHC studies obtained inconsistent results. Müller et al. [2] reported an association of class I HDAC expression with HR status. They found a significant correlation between HDAC1-positive status and HR-positive status and a significant association of HDAC 2 and HDAC3 with a negative HR status. In contrast, Krusche et al. [9] observed a significant correlation between increased expression of HDAC3 and HR-positive tumors. We did not observe any significant associations between HR status and the expression of HDAC1, HDAC2, and HDAC3. On the other hand, a positive correlation between HDAC6 expression and HR status was observed in our study, and Zhang et al. [12] also reported higher HDAC6 expression in HR-positive cases, although they failed to demonstrate statistical significance. The inconsistent, conflicting results regarding the correlation of HDACs and HR may suggest that other regulatory factors exist between these two parameters. Although we did not observe a positive correlation between HDAC1 expression and HR status, we did detect increased expression of HDAC1 in luminal A tumors.
The molecular interactions and associations between HDACs and HER2 have not been well documented in previous reports. Several studies have shown that HDACIs can significantly enhance trastuzumab-induced growth inhibition and apoptosis in erbB-2-overexpressing breast cancer cells, suggesting an association between HER2 and HDACs [10,11,19]. Müller et al. [2] reported that high HDAC2 expression was significantly correlated with overexpression of HER2, and in vitro studies have detected an association of HDAC6 and HER2 in oncogenic tumorigenesis [20]. In the present study, high expression of HDAC1 was found to be significantly associated with a negative HER2 status, but there was no correlation between high HDAC2 expression and HER2 status. High HDAC6 expression was significantly correlated with HER2 amplification in our study, and the high HDAC6 expression observed in luminal B type tumors can be explained by the positive correlation of HDAC6 with HR and HER2 amplification.
HDACs have been investigated as potential prognostic factors for breast cancer, but there are conflicting data regarding their prognostic value. Although Krusche et al. [9] suggested that elevated HDAC1 expression is correlated with improved survival in small and well-differentiated tumors, in patients with HR-positive tumors, and tumors with a HER2-negative phenotype, Müller et al. [2] did not observe any prognostic significance of HDAC1, HDAC2, and HDAC3 expression. Elevated expression of HDAC6 was initially reported to be correlated with more aggressive forms of breast cancer, and it was regarded as a poor prognostic factor [21]. However, later studies by Zhang et al. [12] and Saji et al. [13] obtained opposite results, and Suzuki et al. [22] found that the expression of HDACs was significantly reduced in association with the progression from a normal ductal epithelium, to ductal carcinoma in situ, to invasive ductal carcinoma, suggesting an inverse correlation of HDAC expression with tumor progression in breast cancers. These conflicting results may be associated with adjuvant therapy after surgery. The guidelines for breast cancer patients in Korea recommend the use of anthracycline-based regimens for patients without nodal metastasis, anthracycline plus taxane-based regimens for patients with lymph node metastasis, antihormonal therapy for patients with ER-positive tumors, and trastuzumab for patients with HER2-positive tumors [23]. Taxanes act by shifting the dynamic equilibrium between tubulin and microtubules to the direction of microtubule assembly; HDAC6 is a regulator of tubulin, which is the target of taxanes [17]. Furthermore, ER-α directly regulates tumor sensitivity to taxanes, primarily by estrogen-induced deacetylation of tubulin [24], and combination therapy with taxanes plus antihormonal therapy may affect HDAC function, resulting in better therapeutic responses in HDAC-positive/ER-positive tumors. Furthermore, the prognostic significance of HDAC6 was highlighted in luminal B tumors (HR-positive, and HER2 positive or high Ki-67 indices). HER2 inhibits the metastasis suppressor RECK via Sp1- and HDAC1 dependent mechanisms [25] and is also regulated by HDAC6 via hsp60 deacetylation [19]. Therefore, the therapeutic response to anti-hormonal therapy and trastuzumab may influence the OS and DFS of patients with ER-positive tumors and luminal B tumors in the present study.
HDACIs are one of the new agents for the treatment of various human cancers, and they showed therapeutic effects through the inhibition of HDAC activity in breast cancer [11,26,27]. One such HDACI, SAHA, results in the acetylation of various proteins, and it was shown to induce hsp90 acetylation, leading to polyubiquitylation and the accumulation of misfolded client proteins, including HER2, AKT, c-Raf, Bcr-Abl, and mutant FLT-3 [19]. Furthermore, HDACIs inhibit the chaperone function of hsp90, resulting in the proteasomal degradation of AKT and c-Raf, two of the most prominent progrowth and prosurvival proteins in cancer cells [28]. In addition, HDACIs suppress ER-α expression, but promote ER-β expression, resulting in anticancer activity toward breast cancer [29], and Munster et al. [30] reported an improved response to a combination of vorinostat and hormone therapy in metastatic breast cancers.
Our study has several limitations; this cohort was composed of a limited number of patients with different follow-up durations, most of the tumors in our study were of a less advanced stage, and only a small number of patients showed recurrence or death from their cancers. Although we have observed the prognostic significances of HDACs in some subgroups, the analyzed populations of these groups were small for multivariate analysis. Therefore, multivariate analysis was not performed for luminal B tumors, and a few traditional prognostic factors, including pT stage and nodal status, did not correlate with survival in ER-positive tumors. In addition, although the expression levels of HDACs were well correlated with each other, each HDAC subtype showed different associations with the clinical parameters and had different prognostic values. Therefore, an additional study is needed with a larger number of patients and with longer follow-up durations. Additionally, the association of HDACs with therapeutic effects, including anti-hormonal therapy and HER2-targeted therapy, should be clarified in a large standardized patient group in the future.
In summary, we identified a correlation between HDACs and HR and HER2 in breast cancer via IHC analysis. Elevated expression of HDAC6 was significantly correlated with HER2 amplification and HR expression, and HDAC1 expression was higher in tumors without HER2 amplification. Tumors exhibiting high HDAC1 expression were closely associated with the luminal A phenotype, and luminal B tumors expressed HDAC6 more frequently compared with other types of tumors. HDAC1 and HDAC6 expression were positively correlated with a prolonged OS in ER-positive tumors, and the prognostic significance of HDAC6 was highlighted in tumors with a luminal B subtype. Elevated expression of HDAC2 resulted in good OS in ER-negative tumors.
The positive correlation of HDAC1 and HDAC6 expression with prolonged OS was highlighted in ER-positive tumors of the breast. These findings suggest an association of HDAC activity with other clinical factors and indicate its prognostic value in HR-positive breast cancers.
Figures and Tables
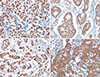 | Figure 1Immunohistochemical staining of histone deacetlylases (HDACs) in invasive ductal carcinomas (×400). (A) HDAC1 and (B) HDAC2 were expressed in the nuclei of tumor cells. (C) HDAC3 was observed in both the cytoplasm and the nuclei. (D) HDAC6 staining was observed in the cytoplasm. |
 | Figure 2Statistical analysis of histone deacetylase (HDAC) expression and survival using the Kaplan-Meier method. (A) High expression of HDAC1 was positively correlated with good overall survival (OS) in the estrogen receptor (ER)-positive group (long-rank test, p=0.017). (B) High expression of HDAC2 was associated with prolonged OS in ER-negative tumors (long-rank test, p=0.048). (C) In patients with ER-positive tumors, the expression of HDAC6 was significantly associated with increased OS (log-rank test, p=0.029). (D) In luminal B tumors, HDAC6 expression predicted good OS (log-rank test, p=0.001). (E) In patients with ER-positive tumors, the expression of HDAC6 was significantly associated with better disease-free survival (log-rank test, p=0.021). (F) HDAC6 expression also predicted prolonged disease-free survival in luminal B tumors (log-rank test, p=0.001). |
Table 2
Immunohistochemical expression of HDAC1, HDAC2, HDAC3, and HDAC6 in invasive ductal carcinomas
References
1. Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006; 11:1–8.

2. Müller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, et al. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer: overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013; 13:215.
3. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001; 61:7025–7029.
5. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006; 6:38–51.

6. Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009; 280:168–176.

7. Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol. 2009; 625:131–142.

8. Longworth MS, Laimins LA. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene. 2006; 25:4495–4500.

9. Krusche CA, Wülfing P, Kersting C, Vloet A, Böcker W, Kiesel L, et al. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005; 90:15–23.

10. Kim YJ, Greer CB, Cecchini KR, Harris LN, Tuck DP, Kim TH. HDAC inhibitors induce transcriptional repression of high copy number genes in breast cancer through elongation blockade. Oncogene. 2013; 32:2828–2835.

11. Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res. 2009; 69:8403–8411.

12. Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004; 10:6962–6968.

13. Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005; 24:4531–4539.

14. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131:18–43.

15. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750.

16. Min SK, Koh YH, Park Y, Kim HJ, Seo J, Park HR, et al. Expression of HAT1 and HDAC1, 2, 3 in diffuse large B-cell lymphomas, peripheral T-cell lymphomas, and NK/T-cell lymphomas. Korean J Pathol. 2012; 46:142–150.

17. Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002; 417:455–458.

18. Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003; 115:727–738.

19. Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005; 11:6382–6389.

20. Scott GK, Marx C, Berger CE, Saunders LR, Verdin E, Schäfer S, et al. Destabilization of ERBB2 transcripts by targeting 3' untranslated region messenger RNA associated HuR and histone deacetylase-6. Mol Cancer Res. 2008; 6:1250–1258.

21. Yoshida N, Omoto Y, Inoue A, Eguchi H, Kobayashi Y, Kurosumi M, et al. Prediction of prognosis of estrogen receptor-positive breast cancer with combination of selected estrogen-regulated genes. Cancer Sci. 2004; 95:496–502.

22. Suzuki J, Chen YY, Scott GK, Devries S, Chin K, Benz CC, et al. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res. 2009; 15:3163–3171.

23. Chung J, Noh H, Park KH, Choi E, Han A. Longer survival in patients with breast cancer with cyclin d1 over-expression after tumor recurrence: longer, but occupied with disease. J Breast Cancer. 2014; 17:47–53.

24. Tokuda E, Seino Y, Arakawa A, Saito M, Kasumi F, Hayashi S, et al. Estrogen receptor-alpha directly regulates sensitivity to paclitaxel in neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2012; 133:427–436.

25. Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn JS, Lee HY, et al. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep. 2011; 25:1677–1681.

26. Huang L, Pardee AB. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol Med. 2000; 6:849–866.

27. Shin JA, Han G, Kim HJ, Kim HM, Cho SD. Chemopreventive and chemotherapeutic effect of a novel histone deacetylase inhibitor, by specificity protein 1 in MDA-MB-231 human breast cancer cells. Eur J Cancer Prev. 2014; 23:277–285.

28. Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, et al. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003; 2:971–984.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download