Abstract
Since recurrent bilateral breast infection due to nontuberculous mycobacterium (NTM) is rare, its diagnosis is easily overlooked; in addition, complete recovery is often difficult to achieve. We report a case of recurrent bilateral infection in a 35-year-old woman who had completed treatment for NTM. Although various infectious diseases show similar clinical conditions and imaging findings, recurrences should raise suspicion of NTM infection, and this possibility should be considered in differential diagnoses.
Mastitis is a common disease with both infectious and noninfectious causes. Most cases occur during lactation and are caused by Staphylococcus aureus and Streptococcus species; parasites and Mycobacterium tuberculosis have rarely been reported to cause breast infections [1,2,3]. Noninfectious inflammations include plasma cell, granulomatous, and lymphocytic mastitis [1].
Recurrent mastitis and breast abscesses may be due to delayed, incomplete, or inappropriate therapy as well chronic Staphylococcus infections [4]. They may also be due to underlying breast lesions. However, the disease is clinically and radiologically very difficult to diagnose. We report a case of recurrent bilateral mastitis shown pathologically to be due to nontuberculous mycobacterial (NTM) infection.
A 35-year-old Korean woman admitted to the in-patient clinic of our institution presented with swelling, pain, and rigidity of the left breast that began 4 days previous. She reported a fever that had lasted 1 day. Physical examination revealed a palpable tender mass, erythema, and swelling in the upper half of the left breast, extending to the nipple. There was no nipple discharge or pyrexia. A clinical impression of mastitis was made. She had no previous history of trauma, operation, or family history of breast cancer; further, she was not immunocompromised.
Prior to admission, the patient had a history of recurrent breast infections beginning in July 2007. She was treated for mastitis of her right breast with pus formation, and she made complete recovery. The second infection occurred 8 months later, in March 2008. She underwent breast biopsy for a mass on her right breast, and a fluid sample was cultured. Based on the culture results, the lesion was diagnosed as an NTM infection, and the patient was treated with combined antibiotic therapy for 12 months. The third infection appeared 9 months after the last treatment, in December 2009. She was admitted for a palpable mass in the left breast. The patient underwent biopsy for this lesion as well, which was also diagnosed as an NTM infection. After 4 months of combined antibiotic therapy, she made a complete recovery. The patient was admitted to the hospital for her fourth mastitis event.
We performed routine breast ultrasonography, which revealed a 2.8 cm, ill-defined, partially multilobulating contoured mass with heterogeneous internal echogenicity in the subareolar area, with diffuse edema of the left breast (Figure 1). No significant vascularity was observed. Several small lymph nodes less than 1 cm in short diameter were visible in the left axilla level I.
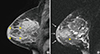
The patient also underwent bilateral dynamic contrast-enhanced magnetic resonance imaging of the affected breast to further evaluate the extent of the lesion and the potential for hidden malignancies (Figure 2). Dynamic contrast-enhanced magnetic resonance imaging revealed a 2.4×2.4 cm iso- to high-signal intense mass on T2 WI and iso-intense on T1 WI, which showed peripheral rim enhancement after contrast injection. Associated findings included diffuse parenchymal enhancement of the upper outer to central portion of the left breast with thickening of the overlying skin. This lesion appeared to be an abscess associated with mastitis.
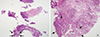
To exclude the possibility of hidden malignancies, a biopsy was performed. Considering the previous histopathologic findings in 2008, 2010, and 2012, granulomatous mastitis was suspected (Figure 3), with no acid-fast bacilli visible on Ziehl-Neelsen staining. Although we could not confirm the identity by specimen culture, paraffin-embedded tissue samples were positive for NTM by polymerase chain reaction. Based on the clinical course of recurrent infections over 5 years, previous histopathologic findings, and positive polymerase chain reaction results, a clinical diagnosis of NTM infection was made. Clarithromycin (1,000 mg/day) was administered in combination with ciprofloxacin (1,000 mg/day) for 1 year. Because the lesion progressed to pus formation, surgical drainage was also performed. The patient underwent a follow-up breast ultrasonography after 1 month that showed lesion improvement. She has been followed up for 1 year with no clinical or radiological evidence of recurrence.
Rarely, NTM organisms can cause breast infections [2,3]. NTM infections may be acute, subacute, or late-onset. Interestingly, previous reports have typically described bilateral NTM breast infections. The most common organisms found in these infections included Mycobacterium fortuitum, M. avium, M. abscessus, and less commonly, M. chelonae [2]. NTM should be considered as a source of infection when standard bacterial culture results are negative.
Initial treatment for infectious and noninfectious mastitis Bincludes appropriate antimicrobial therapy. Generally, patients are administered antibiotics at an early stage without an observation period. It can be difficult to differentiate noninfectious and infectious mastitis at early stages; hence, antibiotics are used in either case. When M. tuberculosis (TB) is suspected, anti-TB therapy may be required. A lack of response to anti-TB therapy or a diffusely deformed breast with draining sinuses may require surgical intervention [5].
Once an NTM organism is isolated by culture, the infection should be treated with targeted combined antibiotic therapy. The most commonly reported cause of skin and soft tissue disease in NTM infections is M. fortuitum. Several published studies have reported M. fortuitum to be associated with breast infections. The majority reported an association between onset of infection and breast implants or reconstructive surgery [6,7,8,9]. They recommended removal of the infected implant in addition to appropriate antimicrobial therapy for the management of implant-associated NTM infections [2]. For postoperative wound infections, the removal of implants or infected foreign bodies was required. Patients treated according to in vitro susceptibilities of isolated colonies have shown good results in a previous study [10]; these targeted therapies are considered essential for infection management and antimicrobial stewardship. A primary panel of drugs for susceptibility testing may include amikacin, cefotoxin, ciprofloxacin, clarithromycin, doxycycline, imipenem, and a sulphonamide [10].
However, few studies have reported NTM breast infections not associated with implants [11]. To our knowledge, there has been only one published report of spontaneous breast abscess due to M. fortuitum [12]; another report described infection following nipple piercing [13,14]. When M. tuberculosis complex species are suspected as the source of infection, targeted antibacterial therapy is recommended [11].
Our case demonstrated continuous recurrent infections despite appropriate treatments for NTM infection. Although we could not confirm the diagnosis by culture, the initial biopsy requested by the regular physician was positive for NTM. The patient was not immunocompromised and had no history of breast surgery, including implant insertion. Therefore, the exact cause of the recurrent NTM infection could not be determined. In conclusion, although it is difficult to differentiate from other causes due to nonspecific imaging findings, NTM should be considered for breast infections that recur despite standard antibiotic therapy.
Figures and Tables
Figure 1
B-mode gray scale ultrasonography of the left breast. (A) Ultrasonogram shows an approximately 2.8 cm ill-defined and partially multilobulating contoured mass with heterogeneous internal echogenicity in the subareolar area. (B) Additional findings of diffuse edematous changes in the left breast, with marked heterogeneous underlying parenchymal echogenicity and skin thickening.
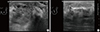
Figure 2
Magnetic resonance imaging: sagittal view of the left breast. (A) Contrast-enhanced magnetic resonance imaging shows an ill-defined and partially multilobular contoured lesion with rim-like enhancement in the subareolar area of the left breast (arrowheads) with additional diffuse parenchymal enhancement. The lesion (arrowheads) visible in the subareolar area correlates to the lesion detected by B-mode gray scale ultrasonography (Figure 1). This lesion appeared to be an abscess consistent with mastitis (1.5-T MR scanner, 3D fat-suppressed T1-weighted Gradient Echo Sequences; contrast injection of 0.2 mL/kg gadodiamide [OmniscanTM; GE Healthcare] was administered by manually followed by a 20-mL saline flush). (B) T2-weighted image showing high signal intensity diffuse edematous changes with skin thickening (arrows) and an ill-defined high signal intensity lesion in the subareolar area (arrowhead), correlated with the lesion with rim-like enhancement.
References
1. Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J. 2009; 15:367–380.

2. Chirra A. Nontuberculous Mycobacterium infection of the breast. Proceedings of UCLA Healthcare. Accessed December 4th, 2013. Available from: http://www.med.ucla.edu/modules/wfsection/article.php?articleid=567.
3. Macadam SA, Mehling BM, Fanning A, Dufton JA, Kowalewska-Grochowska KT, Lennox P, et al. Nontuberculous mycobacterial breast implant infections. Plast Reconstr Surg. 2007; 119:337–344.

4. Abdelhadi MS, Bukharie HA. Breast infections in non-lactating women. J Family Community Med. 2005; 12:133–137.
5. Tewari M, Shukla HS. Breast tuberculosis: diagnosis, clinical features & management. Indian J Med Res. 2005; 122:103–110.
6. Coney PM, Thrush S. Cutaneous Mycobacterium fortuitum complicating breast reconstruction. J Plast Reconstr Aesthet Surg. 2007; 60:1162–1163.

7. Vinh DC, Rendina A, Turner R, Embil JM. Breast implant infection with Mycobacterium fortuitum group: report of case and review. J Infect. 2006; 52:e63–e67.

8. Rimmer J, Hamilton S, Gault D. Recurrent mycobacterial breast abscesses complicating reconstruction. Br J Plast Surg. 2004; 57:676–678.

9. Haiavy J, Tobin H. Mycobacterium fortuitum infection in prosthetic breast implants. Plast Reconstr Surg. 2002; 109:2124–2128.

10. Medical Section of the American Lung Association. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Am J Respir Crit Care Med. 1997; 156:S1–S25.
11. Betal D, Macneill FA. Chronic breast abscess due to Mycobacterium fortuitum: a case report. J Med Case Rep. 2011; 5:188.

12. Cooke FJ, Friedland JS. Spontaneous breast abscess due to Mycobacterium fortuitum. Clin Infect Dis. 1998; 26:760–761.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download