Abstract
Purpose
The aim of this study is to describe our initial experience with magnetic resonance (MR)-guided biopsy and to determine the malignancy rate of additional lesions identified by MR only in Korean women with breast cancer.
Methods
A retrospective review identified 22 consecutive patients with breast cancer who had undergone MR-guided vacuum-assisted biopsies (VAB) of MR-only identified lesions from May 2009 to October 2011.We evaluated the rate of compliance, the technical success for MR-guided VAB and the MR imaging findings of the target lesions. VAB histology was compared with surgical histology and follow-up imaging findings.
Results
The biopsy recommendations for MR-only identified lesions were accepted in 46.8% (22/47) of patients. One of 22 procedures failed due to the target's posterior location. Among 21 MR-guided VAB procedures, the target lesions were considered as a mass in 12 cases and a nonmass enhancement in nine cases. VAB histology revealed malignancies in 14% (3/21) of cases, high-risk lesions in 24% (5/21) and benign lesions in 62% (13/21). Eleven cases (52%, 11/21) had a positive surgical correlation, and one of them was upgraded from atypical ductal hyperplasia to invasive ductal carcinoma. In the remaining 10 lesions, follow-up breast ultrasound and mammography were available (range, 15-44 months; mean, 32.1 months) and did not show suspicious lesions. The final malignancy rate was 19% (4/21).
Contrast-enhanced magnetic resonance (MR) imaging of the breast has high sensitivity (90%-99%) for invasive cancers [1,2]. It is more sensitive for detecting breast cancers than mammography or ultrasound (US) [3]. Breast MR imaging enables the identification of additional malignancies that are mammographically and ultrasonographically occult in approximately 10% to 20% of patients with a malignant breast lesion [4]. However, the reported specificity of MR imaging varies, ranges from 37% to 97% [5]. A recent meta-analysis documented the pooled-weighted specificity to be 72%, with a 95% confidence interval of 67% to 77% [3]. Because of overlapping morphologic structures and the enhancement kinetics of benign and malignant lesions, MR has limited value [5,6,7,8,9]. Given this overlapping of morphological structures, when additional suspicious breast lesions are detected on MR imaging, histopathological analysis is required. The rate of MR-only detected additional lesions has been reported to range from 16% to 51% [10,11,12]. Based on the specific circumstances and the degree of suspicion, these lesions are managed with observation and second-look US or MR-guided intervention. Occasionally, surgeons convert a planned breast-conserving surgery to mastectomy based on evidence obtained from MR alone, and the mastectomy ultimately turns out to be fruitless. A less-invasive biopsy technique would help prevent unnecessary surgical biopsies and associated morbidity risks. Therefore, MR-guided biopsy is essential for broader-use breast MR.
Technological innovations allow minimally invasive diagnostic procedures using core needle biopsy and vacuum-assisted biopsy (VAB), even under MR guidance [13,14,15]. However, the use of MR-guided VAB is not routinely practiced in Korea, and there is only one article describing freehand MR-guided VAB in only five patients [16]. Although the freehand technique is feasible, the use of the compression grid system is much easier and results in a shorter procedure time due to more predictable needle placement [16].
We reviewed our initial experience with MR-guided VAB using the compression grid system and determined the malignancy rate of additional lesions identified by MR-only in Korean women with breast cancer.
This retrospective study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2014-02-073).
From May 2009 to October 2011, breast cancer surgeries were performed on 3,561 patients at our institution. Of them, 3,208 patients underwent preoperative breast MR imaging. In 234 of the 3,208 patients (7%), additional suspicious lesions were detected and the location of lesions could affect the method of the breast cancer surgery. Because the surgeons prefer US-guided procedures due to the lack of wait time, we considered second-look US ahead of MR-guided VAB to verify the lesion's identity; MR-guided VAB was only recommended when it was difficult to localize the lesion with US. Therefore, second-look US was recommended in 187 patients (80%, 187/234) and MR-guided breast VAB was recommended in 47 patients (20%, 47/234). Among them, 22 patients (9%, 22/234) underwent MR-guided breast VABs. All patients were Korean. Figure 1 shows a flowchart for the 3,208 patients who underwent preoperative breast MR imaging.
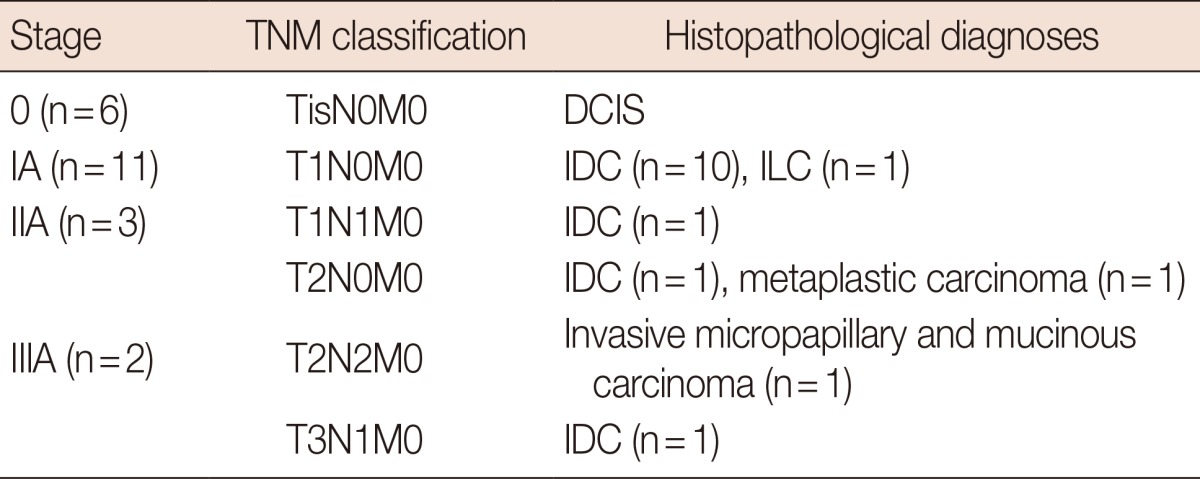
The index cancers were documented pathologically prior to the definitive therapy. The TNM staging and pathologic diagnoses of the index cancers are summarized in Table 1. The age of the 22 patients ranged from 36 to 62 years (mean age, 45.9 years).
Diagnostic breast MR imaging was performed using a 3.0-T system (Achieva; Philips Medical Systems, Best, The Netherlands). All of the patients were examined in the prone position using a dedicated surface breast coil (Sense-Breast-7; Philips Medical Systems). The MR imaging protocol included an axial or sagittal T1-weighted 3D turbo field-echo sequence (T1 high-resolution isotropic volume examination, THRIVE; Philips Healthcare, Best, The Netherlands) with an interval of 1 minute before and 7 times after contrast administration. For contrast enhancement, a 0.1 mmol/kg bolus of gadobutrol (Gadovist; Bayer Healthcare Pharmaceuticals, Berlin, Germany) was injected, followed by a 10-mL saline flush. MR scanning was performed with a minimum repetition time and echo time (4.4/1.6 ms for the axial images; 3.6/1.4 ms for the sagittal images), a 12-flip angle, a 25-34 cm field of view, 1.5 mm sections with no gap, and a matrix size of 376-492×309-492 for the axial images and 312×312 for the sagittal images.
All biopsies were performed by one of two radiologists with 13 years and 6 years, respectively, of specialized experience in breast imaging and imaging-guided breast intervention. They observed experienced radiologists taking VAB more than once, and undertook a phantom study with pork before the biopsy. The biopsies were performed using the same type of MR unit and set-up used for diagnostic MR imaging; a patient in the prone position supported with a dedicated biopsy compression device, and a commercially available grid-localizing system (Biopsy Positioning Device Model MR-BI-160, MRI Devices; GE Healthcare, Little Chalfont, UK) (Figure 2).
A vitamin E capsule was taped near the expected lesion site to serve as a fiducial marker for determining the lesion location, and a sagittal T1-weighted 3D turbo field-echo sequence was obtained. A contrast agent (0.1 mmol/kg bolus of gadobutrol (Gadovist; Bayer Healthcare Pharmaceuticals, Berlin, Germany) was injected and flushed with 10 mL of saline. Postcontrast images were obtained 1 minute after contrast administration. After reviewing the images on the console, a cursor was placed over the fiducial marker and the lesion on the monitor. The differences between the horizontal (dorsal-ventral, x), vertical (cranial-caudal, y), and depth (medial-lateral, z) coordinates of the fiducial marker and the lesion were calculated based on the spatial relationship between the lesion, vitamin E marker, and grid lines (Figure 3).
The patient was withdrawn from the magnet with her breast remaining in compression. After cleansing the skin overlying the lesion site, local anesthesia was administered. The appropriate location for the needle guide was selected. The stylet and introducer were then placed through the needle guide, and a scalpel incision was made in the skin at the site of the tip of the stylet. After the insertion of the stylet introducer assembly up to the appropriate depth (z), the stylet was removed from the breast, leaving the introducer within, and was replaced with the obturator to assist in MR imaging confirmation of the location. The sagittal MR imaging was obtained with the same sequence to document the location of the obturator. To find the tip of the inserted obturator, we reviewed the sagittal MR images with cine mode on the MR console. To see the exact location of the tip, axial images were obtained again. After confirmation of the needle position with MR imaging and removal of the obturator, the 9 G VAB device (Automated Tissue Excision and Collection Breast Biopsy System; Suros Surgical Systems, Indianapolis, USA) was inserted into the introducer. Usually 12 to 18 biopsy specimens were obtained and the biopsy device was removed, followed by the obturator reinsertion. Postbiopsy MR imaging was performed to determine if the lesions had been sampled. We regarded a dark defect and hematoma as evidence of lesion sampling. No clip was inserted. If the lesions were to be surgically removed, preoperative US-guided tattooing localization was performed for post-VAB changes within a week of MR-guided biopsy.
MR imaging findings of the target lesions were retrospectively analyzed based on the original radiologic reports. The findings included morphologic features (mass or nonmass enhancement, margin for the mass, distribution for the nonmass enhancement) [17,18], kinetic features (washout or no washout), maximum size, and location relationship with the index lesions according to the Breast Imaging Reporting and Data System [19]. Because the targeted mass was usually small, we described the mass margin as either smooth or nonsmooth. Rim enhancement was also classified into nonsmooth margin characteristics. The distribution of nonmass enhancement was described as a segmental, linear, or focal pattern.
The VAB and surgical histology were compared. The VAB histology was used to classify benign, high-risk, and malignant lesions. Radial scars, flat epithelial hyperplasia, and any kind of lesions associated with cellular atypia including atypical ductal hyperplasia (ADH) were regarded as high-risk lesions. All of the patients with MR-VAB-detected additional malignant lesions underwent surgery, including for the additional sites. For the high-risk lesions and benign lesions, if the VAB histologic results were discordant, we recommended removal of the lesions with surgery. Since all of the breast cancer patients were operated on in our hospitals, routine postoperative follow-up protocols were applied in all cases, consisting of biannual US and annual mammography during 5 years.
Among the 47 MR-guided VAB recommendations, 22 patients (47%, 22/47) underwent MR-guided breast VABs. In the remaining 25 patients, the surgeons did not accept the MR-guided VAB recommendations due to a tight operation schedule. Second-look US was performed in 18 patients, and 16 patients (34%, 16/47) underwent US-guided tattooing localization for potentially concordant lesions. In the other cases, MR-guided needle localization was performed in one patient (2%, 1/47), mammography-guided needle localization was performed for a presumed lesion in one patient (2%, 1/47), and mastectomy or wide excision were performed in two patients (4%, 2/47). Imaging surveillance was performed to confirm the lesion's identity in five patients (10%, 5/47) (Figure 1).
Twenty-two MR-guided biopsies were attempted in 22 patients. Among them, one case failed due to an overly posterior location of the lesion. The lesion was a nonmass enhancement in the mid-outer region of the right breast. It measured 2.1 cm in the maximum anteroposterior diameter and the distance between the lesion and the chest wall was 0.5 cm. Because this lesion was a faint, gradually enhanced focal area, the surgeon left it untouched and follow-up MR imaging obtained 6 months later revealed disappearance of the lesion. Finally, 21 procedures were completed. The radiologists confirmed the disappearance or decrease of the enhanced target lesions on postbiopsy MR images. No complications such as bleeding, infection or vasovagal reaction occurred during MR-guided VAB, and no 3T-MR related artifacts or difficulties in the biopsy procedure developed. The total time for the biopsy was 40 to 65 minutes.
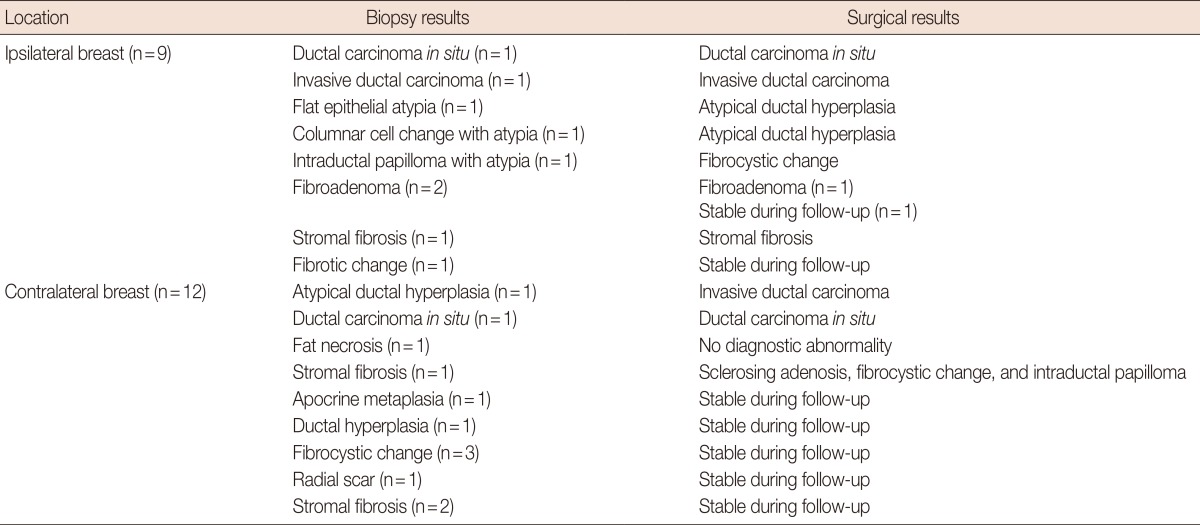
After MR-guided biopsy, 11 patients were operated upon including the MR-guided biopsy site. Because the cost of postbiopsy metallic markers is not covered in our country, we could not insert markers at the VAB site. Instead, we performed US-guided tattooing localization with 0.5 to 1 cc of a charcoal suspension for the biopsy site. In 10 of 11 cases, preoperative US-guided tattooing was successfully achieved for the overt VAB-related hematoma and echogenic air collection (Figure 4) and in one case, tattooing was performed at the approximate area because the biopsy site was not identified.
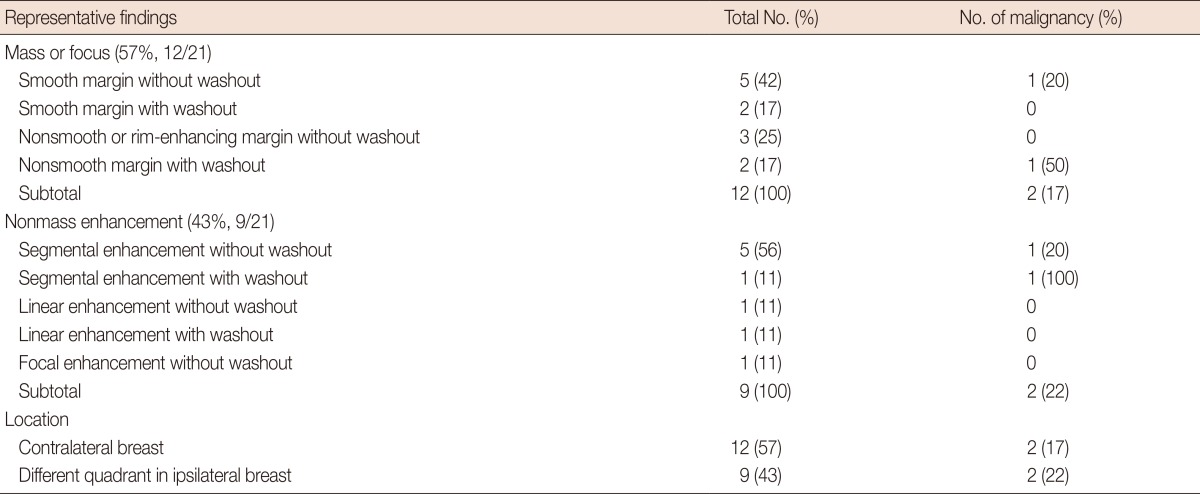
Twenty-one MR-guided biopsies were performed. Lesions were classified as a "mass" in 12 cases (12/21, 57%) and a "nonmass enhancement" in nine cases (9/21, 43%) (Table 2). Even though one lesion had a 0.3-cm focus, we classified this lesion as a mass. Six lesions (6/21, 29%) showed washout kinetics and the rest (15/21, 71%) revealed gradual enhancement. Two malignant lesions found among the masses included a 0.9-cm-sized irregular mass with washout and a 0.5-cm-sized smooth mass without washout, which were confirmed as invasive ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS), respectively. Two malignant lesions were found among the nonmass enhancement. A segmental enhancement lesion without washout was confirmed as DCIS, and a segmental enhancement lesion with washout was revealed as ADH initially, but was upgraded to IDC at surgical excision. The positive predictive value of the MR findings was 33% in segmental enhancement (2/6), 33% in washout kinetics (2/6), 20% in an irregular mass (1/5), and 14% in a smooth mass (1/7). The combination of morphologic and kinetic suspicious features gave a high positive predictive value (segmental enhancement with washout, 100%; irregular mass with washout, 50%). None of the linear or focal nonmass enhancements were malignant.
The mean size of the mass was 0.9 cm (range, 0.3-3.2 cm) and the nonmass enhancement was 2.6 cm (range, 1.2-5.5 cm) on the MR images. All masses were smaller than 1 cm, except for one that was 3.2 cm in size.
Twelve lesions (12/21, 57%) were located in the contralateral breast to that of the primary breast cancer and nine lesions (9/21, 43%) were located in a different quadrant of the same breast (Table 2).
Histologic diagnosis of the VAB specimens was malignant for three lesions (2 DCIS and 1 IDC), five high-risk lesions (1 ADH, 1 columnar cell change with atypia, 1 flat epithelial atypia, 1 intraductal papilloma with atypia, and 1 radial scar) and 13 benign lesions. Two malignancies, three high-risk and four benign lesions were located in a different quadrant of the same breast, and one malignancy, two high-risk and nine benign lesions were located in the contralateral breast of the primary breast cancer (Table 3). The rate of malignancy was 14% (3/21) and the rate of high-risk lesions was 24% (5/21) based on the VAB histologic results.
Surgery was performed in 11 cases: three malignant, four high-risk, and four benign lesions. Among them, one of four high-risk lesions was upgraded from ADH to IDC (25% histologic underestimation rate) and three malignant lesions had concordant surgical histological results. The final malignancy rate of the VAB lesions was 19% (4/21).
The patients with nine benign and one high-risk lesion who did not undergo subsequent surgery received follow-up care consisting of an annual mammography and semiannual US; the mean follow-up period was 32.1 months (range, 15-44 months). There were no malignant lesions detected on follow-up.
We report our initial experience with MR-guided breast VAB using a compression-grid system in preoperative breast cancer patients. In our patient series, MR-guided VAB was technically successful in 95% of patients (21/22). Only one procedure failed, due to the lesion being located too far posteriorly. The rate of malignancy was 14% (3/21) including two DCIS (10%, 2/21), and the rate of high-risk lesions was 24% (5/21). Surgery was performed in four of five cases of high-risk lesions, and one lesion was upgraded to IDC. The frequency of malignancy in this series (19%, 4/21) was lower than that in previous reports of MR-guided VAB in Europe and the United States (malignancy, 18%-61% and in situ carcinoma, 10%-54%) [13,14,15,20,21,22,23,24,25], but it was also lower than that in Japan (malignancy, 33% and in situ carcinoma, 26%) [26]. On average, the breasts of Asian women are small and compact resulting from a lower fat volume compared with that of European or American women. Thus, screening and second-look US detect a considerable number of malignant lesions and the rate of malignancy might be lower in Asian patients than in European or American patients with lesions visible by MR alone [26]. Meanwhile, the low malignancy rate in our study compared with that in a Japanese report might be caused by different patient inclusion criteria. Our study was performed with breast cancer patients who underwent MR imaging preoperatively, whereas their study population had diverse clinical indications for breast MRI such as abnormal findings on mammography, US or positron emission tomography, and postoperative examination [26].
MR-guided breast VAB was recommended in 47 patients, but it was only attempted in 22 patients. MR-guided VAB was not always feasible owing to the limited availability of the MR room, while US-guided or stereotactic-guided core biopsy has been widely used for breast biopsies in our institution. Therefore, many additional suspicious lesions were initially intended for mammography or US-guided biopsy. Only when mammography or second look US-guided biopsy were impossible and inaccurate was MR-guided VAB performed.
The rate of high-risk lesions (24%, 4/21) in this series was higher than that of previous reports of MR-guided VAB, while the underestimation rate (25%, 1/4) was similar to that in previous reports (high-risk lesions, 6%-21% and underestimation rate, 13%-50%) [14,20,24,25,27,28,29]. This may be due to the high pretest probability for breast cancer in our study population that was composed only of cancer patients. In this study, we did not include breast MR imaging for screening purpose.
In our series, the ratio of mass and nonmass enhancement was similar (53% vs. 47%), while Rauch et al. [30] and Han et al. [25] described ratios of 65% vs. 35% and 55% vs. 45%, respectively. Our series showed a slightly higher probability of malignancy in the nonmass enhancement than in the mass enhancement (22% vs. 17%), similar to previous reports (34% vs. 20%). Rauch et al. [30] explained that this trend would be related with a high success rate of second-look US for detecting correlated lesions in masses requiring biopsy. Most enhancing masses are readily identified in second-look US and are biopsied under US-guidance, while the suspicious nonmass enhancement are more likely to undergo MR-guided biopsy.
MR-guided breast biopsy is not popular in Korea; many institutions manage the lesions seen only on MR imaging, depending on the degree of suspicion on MR and US findings, without histologic confirmation. The problems of overtreatment by false positive findings and undertreatment by false negative assessment remain and these problems may undermine the credibility of preoperative MR imaging, cause controversy surrounding the use of MRI. Through the adequate use of MR-guided VAB, we could avoid unnecessary contralateral breast surgery in eight of 12 patients with suspicious lesions in the contralateral breast and unnecessary mastectomy in two of nine patients with suspicious lesions in a different breast quadrant. We think that a more important role of MR-guided breast biopsy is for the confirmation of benign lesions, facilitating scheduled surgery without anxiety and rendering good cosmetic results.
Because this study was conducted during our initial experience, we often had technical difficulties associated with the MR-guided breast biopsy procedure. The most difficult problem is a difference in the morphology of a lesion between the initial diagnostic and prebiopsy MR images. One of the main reasons for this problem is the difference in the imaging plane between these two MR images; prebiopsy images for needle advancement were obtained in the sagittal scan, whereas diagnostic images were often obtained in an axial scan in our study. To solve the issue, the sagittal reformation of the axial images before MR-guided biopsy could have helped with the identification of the target lesions correctly. Another problem was the difficulty in guaranteeing the precise removal of the target lesion, which could be overcome by a sufficiently experienced operator and by using follow-up MR imaging after MR-guided biopsy.
Our study has several limitations. First, the number of cases was small and the degree of suspicion of the target lesions was not very high. Therefore, our results could not reflect the fate of all additional MR-suspicious lesions. Second, it is standard practice to place of a localizing clip following MR-guided biopsy to verify the biopsy cavity and to assess the adequacy of tissue sampling. However, preoperative US-guided tattooing localization was performed for the site of MR-guided biopsy rather than the placement of postbiopsy metallic markers in our study. In this case, confirmation of the lesion retrieval may not be guaranteed when surgery is performed. Third, one of five patients with high-risk lesions did not undergo surgical excision, and follow-up US and mammography were performed instead of MRI. However, all high-risk patients as well as those patients with benign MR-guided VAB pathology received 6-month follow-up mammography and US. The mean duration of follow-up was 32.1 months (range, 15-44 months). Follow-up studies during lasting than 2 years were performed in most patients (7/10), and no malignancy was found. Nevertheless, follow-up MRI should have been performed. Fourth, MR-guided biopsy was not performed for all suspicious lesions and there may be a selection bias in our study. Last, this study was performed retrospectively.
In conclusion, MR-guided biopsy provided a 19% additional malignancy rate and 25% underestimation rate for breast lesions that were detected on breast MR only. MR-guided breast biopsy is a reliable method and is strongly recommended for lesions with abnormal MR enhancement without a corresponding abnormality on mammography or US in preoperative patients with breast cancer.
References
1. Baum F, Fischer U, Vosshenrich R, Grabbe E. Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol. 2002; 12:1087–1092. PMID: 11976850.

2. Helbich TH. Contrast-enhanced magnetic resonance imaging of the breast. Eur J Radiol. 2000; 34:208–219. PMID: 10927162.

3. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008; 246:116–124. PMID: 18024435.

4. Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999; 213:881–888. PMID: 10580970.

5. Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001; 220:13–30. PMID: 11425968.

6. Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997; 203:137–144. PMID: 9122382.

7. Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999; 211:101–110. PMID: 10189459.

8. Orel SG. Differentiating benign from malignant enhancing lesions identified at MR imaging of the breast: are time-signal intensity curves an accurate predictor? Radiology. 1999; 211:5–7. PMID: 10189447.

9. Nunes LW, Schnall MD, Orel SG. Update of breast MR imaging architectural interpretation model. Radiology. 2001; 219:484–494. PMID: 11323476.

10. Kuhl CK, Schmutzler RK, Leutner CC, Kempe A, Wardelmann E, Hocke A, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000; 215:267–279. PMID: 10751498.

11. Brown J, Smith RC, Lee CH. Incidental enhancing lesions found on MR imaging of the breast. AJR Am J Roentgenol. 2001; 176:1249–1254. PMID: 11312189.

12. Teifke A, Lehr HA, Vomweg TW, Hlawatsch A, Thelen M. Outcome analysis and rational management of enhancing lesions incidentally detected on contrast-enhanced MRI of the breast. AJR Am J Roentgenol. 2003; 181:655–662. PMID: 12933456.

13. Liberman L, Morris EA, Dershaw DD, Thornton CM, Van Zee KJ, Tan LK. Fast MRI-guided vacuum-assisted breast biopsy: initial experience. AJR Am J Roentgenol. 2003; 181:1283–1293. PMID: 14573421.
14. Orel SG, Rosen M, Mies C, Schnall MD. MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology. 2006; 238:54–61. PMID: 16304093.

15. Perlet C, Heywang-Kobrunner SH, Heinig A, Sittek H, Casselman J, Anderson I, et al. Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer. 2006; 106:982–990. PMID: 16456807.
16. Choi HY, Kim SM, Jang M, Yun BL, Kim SW, Kang E, et al. MRI-guided intervention for breast lesions using the freehand technique in a 3.0-T closed-bore MRI scanner: feasibility and initial results. Korean J Radiol. 2013; 14:171–178. PMID: 23482868.

17. LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003; 227:856–861. PMID: 12773685.

18. Liberman L, Morris EA, Lee MJ, Kaplan JB, LaTrenta LR, Menell JH, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol. 2002; 179:171–178. PMID: 12076929.

19. Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am. 2002; 40:409–430. PMID: 12117184.

20. Malhaire C, El Khoury C, Thibault F, Athanasiou A, Petrow P, Ollivier L, et al. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol. 2010; 20:1554–1562. PMID: 20119729.

21. Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol. 2005; 185:183–193. PMID: 15972421.

22. Mahoney MC. Initial clinical experience with a new MRI vacuum-assisted breast biopsy device. J Magn Reson Imaging. 2008; 28:900–905. PMID: 18821610.

23. Lehman CD, Deperi ER, Peacock S, McDonough MD, Demartini WB, Shook J. Clinical experience with MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2005; 184:1782–1787. PMID: 15908530.

24. Liberman L, Holland AE, Marjan D, Murray MP, Bartella L, Morris EA, et al. Underestimation of atypical ductal hyperplasia at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007; 188:684–690. PMID: 17312054.

25. Han BK, Schnall MD, Orel SG, Rosen M. Outcome of MRI-guided breast biopsy. AJR Am J Roentgenol. 2008; 191:1798–1804. PMID: 19020252.

26. Tozaki M, Yamashiro N, Sakamoto M, Sakamoto N, Mizuuchi N, Fukuma E. Magnetic resonance-guided vacuum-assisted breast biopsy: results in 100 Japanese women. Jpn J Radiol. 2010; 28:527–533. PMID: 20799018.

27. Perlet C, Heinig A, Prat X, Casselman J, Baath L, Sittek H, et al. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol. 2002; 12:1463–1470. PMID: 12042955.

28. Crystal P, Sadaf A, Bukhanov K, McCready D, O'Malley F, Helbich TH. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted. Eur Radiol. 2011; 21:582–589. PMID: 20839000.

29. Noroozian M, Gombos EC, Chikarmane S, Georgian-Smith D, Raza S, Denison CM, et al. Factors that impact the duration of MRI-guided core needle biopsy. AJR Am J Roentgenol. 2010; 194:W150–W157. PMID: 20093566.

30. Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol. 2012; 198:292–299. PMID: 22268171.

Figure 1
Flowchart for additional suspicious lesions on magnetic resonance imaging (MRI) in patients with breast cancer who underwent preoperative breast MRI and surgeries.
US=ultrasound.
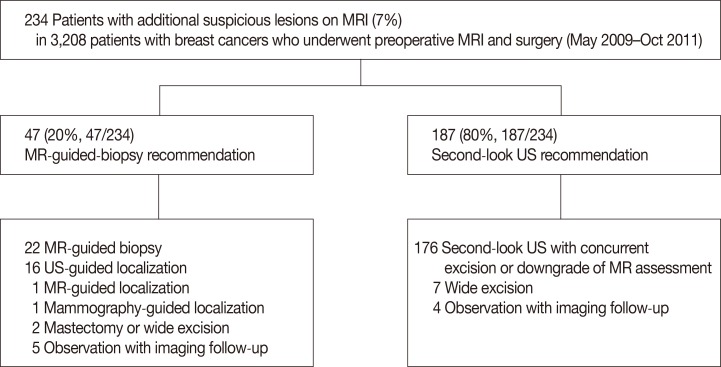
Figure 2
(A, B) Dedicated biopsy compression device and a commercially available compression grid-localizing system (Biopsy Positioning Device Model MR-BI-160, MRI Devices; GE Healthcare).
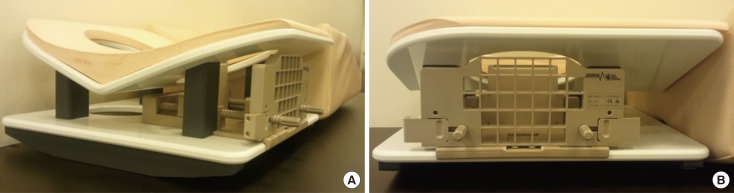
Figure 3
A method of determining the lesion location. (A, B) After reviewing the images on the console, a cursor was placed over the lesion (in a circle) and fiducial marker (in a rectangle) on the monitor. The differences in dorsal-ventral (x), cranial-caudal (y), and medial-lateral (z) direction coordinates of the lesion and fiducial marker were calculated on the basis of the spatial relationship between the lesion, vitamin E marker, and grid lines. The difference in z direction was calculated by a following formula: difference of imaging number of the lesion and fiducial marker×slice thickness of the magnetic resonance images.
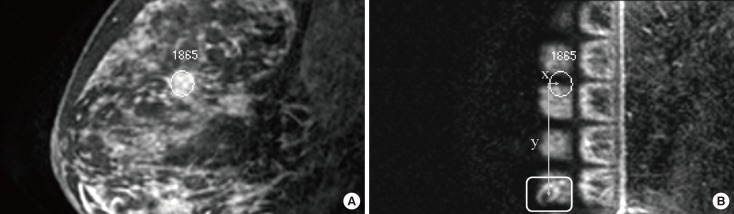
Figure 4
Images of a 49-year-old woman with diagnosed as ductal carcinoma in situ (DCIS) in the left breast. (A) Initial diagnostic contrast enhanced sagittal T1-weighted 3D turbo field-echo image shows segmental enhancing lesion without washout in the mid-outer region of the contralateral (right) breast (arrow). Mammograms and second-look ultrasound did not show clear relation with magnetic resonance (MR) imaging finding. (B) Sagittal prebiopsy MR image with the same sequence reveals the tip of inserted obturator at the targeted lesion (arrow). (C) Axial MR image confirms the exact location of the tip (arrow). (D) Sagittal MR image obtained after vacuum biopsy shows air at anterior to biopsy site and lesion disappearance (arrow). (E) Overt hematoma (arrows) and echogenic air collection (arrowheads) are seen on ultrasound (US). US-guided tattooing for the vacuum-assisted biopsy site was done before the surgery. MR-guided vacuum-assisted biopsy revealed DCIS. Operation after the biopsy confirmed DCIS.
Table 1
TNM staging and histopathological diagnoses of the index cancers in 22 patients who underwent magnetic resonance-guided vacuum-assisted biopsy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download