Abstract
Purpose
Several accelerated partial breast irradiation (APBI) techniques are being investigated in patients with early-stage breast cancer. The present study evaluated the feasibility, early toxicity, initial efficacy, and cosmetic outcomes of accelerated partial breast intensity-modulated radiotherapy (IMRT) for Chinese female patients with early-stage breast cancer after breast-conserving surgery.
Methods
A total of 38 patients met the inclusion criteria and an accelerated partial breast intensity-modulated radiotherapy (APBI-IMRT) plan was designed for each patient. The prescription dose was 34 Gy in 10 fractions, 3.4 Gy per fraction, twice a day, in intervals of more than 6 hours.
Results
Of the 38 patients, six patients did not meet the planning criteria. The remaining 32 patients received APBI-IMRT with a mean target volume conformity index of 0.67 and a dose homogeneity index of 1.06. The median follow-up time was 53 months and no local recurrence or distant metastasis was detected. The most common acute toxicities observed within 3 months after radiotherapy were erythema, breast edema, pigmentation, and pain in the irradiated location, among which 43.8%, 12.5%, 31.3%, and 28.1% were grade 1 toxicities, respectively. The most common late toxicities occurring after 3 months until the end of the follow-up period were breast edema, pigmentation, pain in the irradiated location, and subcutaneous fibrosis, among which 6.2%, 28.1%, 21.9%, and 37.5% were grade 1 toxicities, respectively. Thirty-one patients (96.8%) had fine or excellent cosmetic outcomes, and only one patient had a poor cosmetic outcome.
Whole-breast irradiation (WBI) has become one of the key components in breast conserving therapy. Breast cancer radiotherapeutic methods are continuously updated as new comprehensive therapeutic strategies for breast cancer are developed. After breast conserving surgery, 80% to 90% of local recurrences occur at the site of the original tumor lesion and adjacent regions [1]. Therefore, some investigators have questioned whether it is necessary and appropriate for all patients treated with breast conserving surgery to undergo WBI. As they have suggested, accelerated partial breast irradiation (APBI) has rapidly become the focus of clinical research.
External-beam APBI is one of the most cost-effective therapeutic approaches for APBI [2]. It reduces late-stage tissue fibrosis and fat necrosis, thereby improving cosmetic outcomes compared with other irradiation modes (e.g., interstitial brachytherapy or intraoperative radiotherapy). However, external-beam radiation may subject other healthy tissues, including breast tissue, to a moderate dose of irradiation [3]. Therefore, it is important to develop external-beam radiation techniques that are more conformal.
Intensity-modulated radiotherapy (IMRT) is one of the most rapidly growing therapeutic approaches in recent years. Partial breast IMRT provides a more even and conformal dose coverage than partial breast three-dimensional conformal radiotherapy (3DCRT) or helical tomotherapy [4]. The volume of normal breast tissues irradiated by IMRT is smaller than that irradiated by 3DCRT [5]. Furthermore, IMRT can reduce the dose to the skin [6]. Our dosimetric comparison showed that accelerated partial breast IMRT (APBI-IMRT) could significantly reduce the cardiac radiation dosage compared with WBI [7]. In addition to exposure, it is important to note the cardiotoxicity of radiotherapy [8]. Therefore, patients may benefit more from IMRT as an external-beam APBI method due to its improved dose coverage and protection of normal tissues and organs. The present study focused on understanding the feasibility of APBI-IMRT in Chinese female patients while investigating its initial cosmetic outcomes, toxicity, and clinical efficacy.
Chinese female patients treated with breast conserving surgery for early-stage breast cancer at Sun Yat-Sen University Cancer Center were recruited in this phase II study. The following inclusion criteria were utilized for patient selection: female; unilateral breast cancer; single lesion; undergoing breast conserving surgery; over 18 years of age; pathologically confirmed as having infiltrating cancer (including duct cancer, medullary carcinoma, mucinous adenocarcinoma, and tubular carcinoma); pathology-negative lymph nodes confirmed by axillary lymph node dissection or sentinel lymph node biopsy; American Joint Committee on Cancer (AJCC) stage I or II (pT1N0M0, pT2N0M0) with a primary tumor size ≤3 cm; negative margins under the microscope (>1 cm); complete results including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor family 2 (HER2); no small sporadic calcification in the local lesion, as determined by ipsilateral breast postsurgical molybdenum target photography; no multicentric lesions, as determined by ipsilateral breast postsurgical magnetic resonance imaging; and no contraindications for radiotherapy (i.e., severe heart disease, high blood pressure, rheumatic and immune diseases, or a history of other cancer). All patients and their family members provided signed informed consent, and the present study was approved by the ethics committee of Sun Yat-Sen University Cancer Center (approval number of Institutional Review Board: 2008B030301106).
Patients meeting the inclusion criteria were placed in the supine position on a postural immobilization bracket (Thorawedge™ SIN-301080; Siemens, Munich, Germany). The median interval from surgery to computed tomography (CT) simulation was 4 months (range, 1-5 months). The scanning was performed using a Philips Medical Madison helix CT scanner (Philips, Amsterdam, The Netherlands) with the patient breathing calmly. A metal marker was placed onto the patient's body as a laser reference point for CT positioning. Contiguous 5 mm CT axial images were obtained extending from the larynx to the upper abdomen, including the entire breasts and both lungs. After scanning, the imaging data were transferred into the Pinnacle 7.4f® (Philips Medical Systems, Milpitas, USA) treatment planning system for radiotherapy planning.
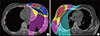
The surgical cavity was delineated under a CT window width of 500 and level of 0, in accordance with the following factors: the metal marker placed during operation; the residual seroma after operation; the interruption and density variations of the breast glands in the CT images; and the surgical cavity shown by ultrasonography in the same position, including the distance between the surgical cavity or interruption of breast glands and certain fixed points on the skin; and the depth, width and height of the cavity. The delineation of the surgical cavity was finally approved by three experienced radiation oncologists (Figure 1).
The clinical target volume (CTV) was delineated by expanding the surgical cavity evenly by 10 mm with modification. The anterior boundary was located within 5 mm under the skin surface with the posterior boundary defined as the surface of the pectoral muscles.
The planning target volume (PTV) was delineated by expanding the CTV evenly by 10 mm. The PTV for evaluation (PTV_EVAL) was formed by modifying the PTV in accordance with the International Commission on Radiation Units (ICRU) 50 and ICRU62 reports, with the anterior boundary at 5 mm under the chest skin and the bottom not exceeding the thorax.
The bilateral lungs, heart, and thyroid were delineated on each slice of the CT image. The ipsilateral and contralateral breasts were contoured including all of the breast tissue in the standard whole breast tangential fields from the inframammary fold to the clavicle in the cranial-caudal direction, with the anterior boundary at 5 mm under the skin and the bottom on the chest wall. External marker wires were placed to indicate the clinical expectations of the external beam tangential field borders as a guide.
The Pinnacle 7.4f® treatment planning system was used for the design of the IMRT plan with a 6 MV photon beam. The prescription dose was 34 Gy/10 fractions/5 days (3.4 Gy/fractions, twice a day) with an interdose interval of more than 6 hours. The design adapted direct machine parameter optimization with five coplanar or noncoplanar fields. A static multileaf collimator was used to modulate the intensity of the complete irradiation in each subfield through the step-and-shoot method. The limitation parameters were calculated and adjusted in accordance with the actual dose to reach the desired dose coverage in the target volume while limiting the dose to the involved organs within the designated range. For each patient, the IMRT dosage was evaluated using the dose-volume histogram (DVH) in accordance with the Radiation Therapy Oncology Group (RTOG)-0319 evaluation criteria [9]. The evaluation criteria for normal organs are listed as follows. For the ipsilateral breast, <25% of the breast volume received the prescription dose, and <50% of the breast volume received ≥50% of the prescription dose. For the contralateral breast, the maximum point dose was <3% of the prescription dose. For the ipsilateral lung, <10% of the lung tissue received 5% of the prescription dose. For the heart, in cases of right breast cancer, <10% of the heart tissue received 5% of the prescription dose, and for cases of left breast cancer, the heart volume receiving 5% of the prescription dose was smaller than that receiving the same dose with conventional whole-breast tangent field irradiation. For the thyroid, the maximum point dose was <3% of the prescription dose.
1) No variations (total coverage): 95% of the isodose surface covered 100% of the PTV_EVAL. All limits of the specified critical normal tissue DVH were met. 2) Minor variations (marginal coverage): 95% of the isodose surface coverage was between 95% and 100% of the PTV_EVAL. There was no portion of the PTV_EVAL receiving <93% of the prescription (isocenter) dose. All limits of the specified critical normal tissue DVH fell within 5% of the guidelines. 3) Major variations (missing): 95% of the isodose surface covered <95% of the PTV_EVAL. A portion of the PTV_EVAL received <93% of the prescription (isocenter) dose.
Treatment plans evaluated as having 'no variations' and 'minor variations' were clinically implemented, and patients whose plans were evaluated as having 'major variations' were withdrawn and those patients were treated with WBI.
The patients were evaluated once per day during the treatment period for radiotherapy-induced acute toxicity. Patients continued to be evaluated at 1 week, 1 month, 2 months, and 3 months posttreatment. After 3 months, patients were evaluated every 3 months in the first year and every 6 months in the second year for postradiotherapy toxicity, tumor recurrence, and metastasis. Chest radiology and breast ultrasonography or molybdenum target photography were performed periodically (generally once per year). Follow-up ended on April 1, 2014.
The conformity index (CI)=VTref/VT×VTref/VRef, where VTref represents the target volume covered by the isodose, VT represents the target volume, and VRef represents the total volume covered by 95% of the isodose. The CI range was 0-1, in which the conformity was better when the CI value was larger. The homogeneity index (HI)=D5/D95, where D5 represents the irradiation dose received by 5% of the PTV-EVAL volume and D95 represents the irradiation dose received by 95% of the PTV-EVAL. The closer to 1 the HI value is, the better the target uniformity will be.
The Harris criteria ( 1979) were used for the evaluation of breast cosmetic outcomes [10]. The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) were used for the evaluation of acute and late toxicity [11]. We evaluated radiotherapy-induced toxicity that arose immediately posttreatment until the end of the last day of follow-up. The obtained values represented the most serious toxicity in patients within these set periods.
A total of 38 patients meeting the inclusion criteria between October 2008 and October 2011 were selected in IMRT planning. Six of the 38 patients (15.7%) were off-protocol due to planning criteria violations and were treated with WBI; the details of the dose constraints of the unsuccessful plans are shown in Table 1 and Figure 2. Therefore, 32 patients received APBI-IMRT.
The clinical pathological information is provided in Table 2. The median age at onset was 43 years (range, 26-65 years). Tumors arose in the upper outer quadrant in 20 of 32 patients (62.5%). Axillary lymph node dissection was performed in 23 of 32 patients (71.9%), with a median number of 15 (range, 5-32) dissected lymph nodes, and 9 of 32 (28.1%) underwent sentinel lymph node biopsy, with a median of 3 (range, 2-5) examined lymph nodes. Of the patients, 28 of 32 (87.5%) received chemotherapy with anthracycline and/or taxane regimens, and the median number of cycles was 6 (range, 6-8 cycles). All ER- and PR-positive patients received endocrine therapy. None of the HER2-positive patients were treated with trastuzumab.
The target volume and normal tissue volume, as well as the doses for the 32 patients in this study are shown in Tables 3 and 4. Among them, 29 patients received coplanar field irradiation, and three patients received noncoplanar field irradiation. The median interval from surgery to IMRT was 4 months (range, 1-5 months). The treatment planning for 18 patients was determined to have no variations, and for 14 patients, it was determined to have minor variations. The average target volume CI was 0.67 (range, 0.49-0.78) and the average dose HI was 1.06 (range, 1.02-1.12). The average ratio between the PTV-EVAL volume and the ipsilateral breast volume was 0.20 (range, 0.07-0.45), and the ratio in six patients was >0.25 (part of the PTV_EVAL target volume was located outside of breast tissues). Three patients with a PTV-EVAL to ipsilateral breast volume ratio of >0.25 were determined to have no variations, and the other three patients were determined to have minor variations.
For all patients, follow-up started from the first day of surgery. One patient was lost to follow-up after 1 year. The overall follow-up rate was 97%, and the median period was 53 months (range, 12-64 months). Through the end of the follow-up period, no patients had local recurrence or distant metastasis, and both the local control rate and the overall survival rate were 100%.
The most acute toxic reactions within 3 months of radiotherapy were erythema, breast edema, pigmentation, and pain in the irradiated location, among which 43.8%, 12.5%, 31.3%, and 28.1%, respectively, were grade 1 reactions. Most late toxicities occurring by the end of the follow-up period were breast edema, pigmentation, pain in the irradiated location, and subcutaneous fibrosis, among which 6.2%, 28.1%, 21.9%, and 37.5%, respectively, were grade 1 reactions (Table 5).
Of the 32 patients, only one patient (3.2%) had a poor cosmetic outcome. All of the remaining 31 patients (96.8%) had a fine or excellent cosmetic outcome; of these, 22 patients (68.7%) were assessed as excellent and nine patients were (28.1%) assessed as fine.
In the present study, we performed APBI-IMRT in Chinese female patients who underwent with breast conserving surgery to test the toxicity, efficacy, and cosmetic outcomes of the procedure. Our results show that APBI-IMRT achieved better target volumes and dose conformity, good cosmetic outcomes, and clinical efficacy as evidenced in the short-term follow-up, with only mild toxicity.
The cosmetic outcomes from APBI are closely correlated with the irradiation volume [12]. Women from Eastern countries have significantly smaller breasts than women from Western countries [13,14]. Therefore, an irradiation mode with better conformity should improve the cosmetic outcomes in breast cancer patients receiving APBI.
In the present study, through the end of the follow-up period, no local recurrences or distant metastases were found in any of the 32 patients. However, since the median follow-up period was only 53 months, it was premature to make conclusions regarding long-term clinical efficacy. A previous study of 199 patients receiving APBI (interstitial brachytherapy) and 199 patients receiving WBI, with a median follow-up period of 9.6 years reported local recurrence rates within 10 years of 5% among those receiving APBI and 4% among those receiving WBI (p=0.48) [15]. When the median follow-up period was extended to 14.5 years, there was no difference in the local recurrence between WBI and APBI (3.8% vs. 5.0%, p=0.40) [16]. Polgár et al. [17] investigated 128 patients receiving APBI (interstitial brachytherapy or local electron beam irradiation) and 130 patients receiving WBI from 1998 to 2004, with a median follow-up period of 66 months. They reported 5-year local recurrence rates of 4.7% for APBI and 3.4% for WBI (p=0.50) and overall survival rates of 94.6% and 91.8% (p>0.05), respectively. Among these patients, 77.6% and 62.9% of the subjects had fine or excellent cosmetic outcomes (p=0.009), respectively. Taken together, these reports demonstrated the potential superior efficacy of radiotherapy and that good cosmetic outcomes could be achieved with APBI after breast conserving surgery. However, large phase III clinical trials are needed to further confirm the clinical efficacy.
In the present study, the acute toxicity and late toxicity were mainly erythema, breast edema, pigmentation, pain in the irradiated location, and subcutaneous fibrosis; no reactions of grade 2 or higher were identified. Most patients had excellent cosmetic outcomes with a relatively short follow-up period. A study has also demonstrated that the incidence of early toxicity (grade 1 and 2) after APBI-IMRT was significantly lesser than that after WBI (5.8% vs. 41%) [18]. Likewise, another study investigated 36 patients treated with APBI-IMRT and found that the most common acute toxicities (grade 1 and 2) were erythema, pigmentation, and pain in the breast or chest wall. No grade 3 or 4 toxicities were reported, while cosmetic outcomes were considered "excellent" or "good" by 94% of patients and 97% of physicians [19]. Leonard et al. [20] investigated 55 patients treated with APBI-IMRT, with a median follow-up period of 10 months. In total, 98.2% of their subjects had fine or excellent cosmetic outcomes. Twenty patients reported grade 1-2 breast pain, two patients reported grade 1 breast edema, and one patient reported angiotelectasis.
Theoretically, IMRT has advantages in APBI. However, Jagsi et al. [12] reported that among 34 patients who received APBI-IMRT under active breathing control with 38.5 Gy/10 fractions and a median follow-up period of 2.5 years, seven patients (21%) showed unacceptable cosmetic outcomes, which were the poorest reported outcomes in recent years. These results indicated that there was strong correlation between cosmetic outcomes and normal breast irradiation volumes. The acceptable and unacceptable cosmetic outcomes for whole-breast V50 were 34.6% and 46.1% (p=0.02), and for whole-breast V100, they were 15.5% and 23.0% (p=0.02), respectively. Since there was a significant correlation in APBI between normal breast irradiation volume and cosmetic outcomes, it is necessary to impose strict limitations on breast doses. IMRT provides more conformal protection for surrounding organs at risk in the target area than 3DCRT. With the help of technologies like active breathing control and image guide radiation therapy in the future, IMRT may be more beneficial for patients.
Currently, the indications for APBI have not been defined. Both the American Society of Radiation Oncology (ASTRO) and the Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology (GEC-ESTRO) have suggested that some patients can be considered for APBI [21,22]. Their suggestion focuses mainly on patients at a low-risk for recurrence. Indications for ABPI remain controversial in patients with early-stage breast cancer after breast conserving surgery [23]. We consider the indications suggested by ASTRO and GEC-ESTRO to be good references for screening patients to receive APBI. However, in the present study, only 3.1% (one patient >60 years of age) of patients were suitable to receive APBI when we followed the age criteria suggested by ASTRO. This could be because the peak age of breast cancer occurrence in Chinese females is 10 years earlier than that in Western females due to the differences in the age of onset between Eastern and Western women. More studies with long-term follow-up are required to determine whether age will make any difference in the prognosis of Chinese females treated with APBI. Furthermore, the doses used in external-beam partial breast irradiation remain controversial. The reported doses in most patients were 25 to 40 Gy, and a single dose was 3.4 to 6.0 Gy [17,24,25,26,27]. However, using radiobiological calculations, the most optimized single dose should be 3.82 Gy [28]. Therefore, the recommended dose was 38.5 Gy/10 fractions, twice a day. The dose mode was 34 Gy/10 fractions in our previous studies on partial breast 3DCRT and the primary results showed that this was a safe and effective dose [14]. Therefore, a dose of 34 Gy/10 fractions was repeatedly used in the present study. A subsequent clinical trial with stepwise dose escalation is under consideration for future studies.
In the present study, 78.1% patients had outer quadrant lesions and the contralateral breast was exposed to a low dose of radiation; in a patient with a tumor located in the upper inner quadrant, the APBI-IMRT plan was assessed as having major variations due to overdosage to the contralateral breast. The contralateral breast dose and the incidence of secondary breast cancer following contralateral breast irradiation should be considered and compared between IMRT and conventional whole breast irradiation; in a dosimetric study with WBI, the IMRT technique did not increase the out-of-field dose in the contralateral breast compared to conventional techniques, which should ultimately translate into a lower secondary breast cancer risk [29]. However, while breast cancer patients may live longer with the improvement of adjuvant treatment, radiotherapy-induced contralateral breast cancer still cannot be ignored. The doses of radiation to contralateral breast should be minimized when using the IMRT technique.
We recognize that there were limitations to our present study. The sample size was small, and a relatively short duration of follow-up was used. In addition, since the respiratory gating system was not employed, the dosimetry might be influenced by the individual's respiration.
In conclusion, the present study demonstrates that accelerated partial breast IMRT is feasible for Chinese female patients after breast conserving surgery with mild toxicity, good clinical efficacy, and superior cosmetic outcomes. The results from additional studies with a larger sample size and longer follow-up period are needed to further confirm its efficacy and long-term radiotherapeutic toxicity in Chinese female patients.
Figures and Tables
Figure 1
Delineation of the surgical cavity (white arrows) based on surgical clips (A), seroma (B), and ultrasound (C).

Figure 2
Dose distribution of two patients off-protocol. (A) Case 23 with tumor located in low inner quadrant, dose of heart and ipsilateral lung is in excess of the evaluation criteria of RTOG-0319. (B) Case 24 with tumor located in upper outer quadrant, dose of uninvolved normal breast is in excess of the evaluation criteria of RTOG-0319.
Notes
This study was supported by a grant from the Sci-Tech Office of Guangdong Province (No. 2008B060600019), the Youth Foundation of the First Affiliated Hospital of Xiamen University (No. XYY2012005), the Education Scientific Research Project of Young Teachers in Fujian Province (No. JB13131) and Medical Scientific Research Foundation of Guangdong Province (No. A2010192).
References
1. Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001; 12:997–1003.

2. Sher DJ, Wittenberg E, Suh WW, Taghian AG, Punglia RS. Partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys. 2009; 74:440–446.

3. Njeh CF, Saunders MW, Langton CM. Accelerated Partial Breast Irradiation (APBI): a review of available techniques. Radiat Oncol. 2010; 5:90.

4. Oliver M, Chen J, Wong E, Van Dyk J, Perera F. A treatment planning study comparing whole breast radiation therapy against conformal, IMRT and tomotherapy for accelerated partial breast irradiation. Radiother Oncol. 2007; 82:317–323.

5. Rusthoven KE, Carter DL, Howell K, Kercher JM, Henkenberns P, Hunter KL, et al. Accelerated partial-breast intensity-modulated radiotherapy results in improved dose distribution when compared with three-dimensional treatment-planning techniques. Int J Radiat Oncol Biol Phys. 2008; 70:296–302.

6. Saibishkumar EP, MacKenzie MA, Severin D, Mihai A, Hanson J, Daly H, et al. Skin-sparing radiation using intensity-modulated radiotherapy after conservative surgery in early-stage breast cancer: a planning study. Int J Radiat Oncol Biol Phys. 2008; 70:485–491.

7. Wu S, He Z, Guo J, Li F, Lin Q, Guan X. Dosimetric comparison of normal structures associated with accelerated partial breast irradiation and whole breast irradiation delivered by intensity modulated radiotherapy for early breast cancer after breast conserving surgery. Clin Transl Oncol. 2014; 16:69–76.

8. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013; 368:987–998.

9. Vicini F, Winter K, Straube W, Wong J, Pass H, Rabinovitch R, et al. A phase I/II trial to evaluate three-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for Stage I/II breast carcinoma: initial report of feasibility and reproducibility of Radiation Therapy Oncology Group (RTOG) Study 0319. Int J Radiat Oncol Biol Phys. 2005; 63:1531–1537.

10. Harris JR, Levene MB, Svensson G, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979; 5:257–261.

11. National Cancer Institute. Common terminology criteria for adverse events v3.0 (CTCAE). 2006. Accessed May 21st, 2008. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
12. Jagsi R, Ben-David MA, Moran JM, Marsh RB, Griffith KA, Hayman JA, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010; 76:71–78.

13. Hepel JT, Tokita M, MacAusland SG, Evans SB, Hiatt JR, Price LL, et al. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2009; 75:1290–1296.

14. Li FY, He ZY, Xue M, Chen LX, Wu SG, Guan XX. Feasibility and acute toxicity of 3-dimensional conformal external-beam accelerated partial-breast irradiation for early-stage breast cancer after breast-conserving surgery in Chinese female patients. Chin Med J (Engl). 2011; 124:1305–1309.
15. Antonucci JV, Wallace M, Goldstein NS, Kestin L, Chen P, Benitez P, et al. Differences in patterns of failure in patients treated with accelerated partial breast irradiation versus whole-breast irradiation: a matched-pair analysis with 10-year follow-up. Int J Radiat Oncol Biol Phys. 2009; 74:447–452.

16. Shah C, Antonucci JV, Wilkinson JB, Wallace M, Ghilezan M, Chen P, et al. Twelve-year clinical outcomes and patterns of failure with accelerated partial breast irradiation versus whole-breast irradiation: results of a matched-pair analysis. Radiother Oncol. 2011; 100:210–214.

17. Polgár C, Fodor J, Major T, Németh G, Lövey K, Orosz Z, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma: 5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007; 69:694–702.

18. Livi L, Buonamici FB, Simontacchi G, Scotti V, Fambrini M, Compagnucci A, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 2010; 77:509–515.

19. Lewin AA, Derhagopian R, Saigal K, Panoff JE, Abitbol A, Wieczorek DJ, et al. Accelerated partial breast irradiation is safe and effective using intensity-modulated radiation therapy in selected early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012; 82:2104–2110.

20. Leonard C, Carter D, Kercher J, Howell K, Henkenberns P, Tallhamer M, et al. Prospective trial of accelerated partial breast intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007; 67:1291–1298.

21. Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009; 74:987–1001.

22. Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol. 2010; 94:264–273.

23. Vicini F, Arthur D, Wazer D, Chen P, Mitchell C, Wallace M, et al. Limitations of the American Society of Therapeutic Radiology and Oncology Consensus Panel guidelines on the use of accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011; 79:977–984.

24. Taghian AG, Kozak KR, Doppke KP, Katz A, Smith BL, Gadd M, et al. Initial dosimetric experience using simple three-dimensional conformal external-beam accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2006; 64:1092–1099.

25. Formenti SC, Rosenstein B, Skinner KA, Jozsef G. T1 stage breast cancer: adjuvant hypofractionated conformal radiation therapy to tumor bed in selected postmenopausal breast cancer patients: pilot feasibility study. Radiology. 2002; 222:171–178.

26. Bourgier C, Pichenot C, Verstraet R, El Nemr M, Heymann S, Biron B, et al. Early side effects of three-dimensional conformal external beam accelerated partial breast irradiation to a total dose of 40 Gy in one week (a phase II trial). Int J Radiat Oncol Biol Phys. 2011; 81:1228–1235.

27. Vicini F, Winter K, Wong J, Pass H, Rabinovitch R, Chafe S, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010; 77:1120–1127.

28. Cuttino LW, Todor D, Pacyna L, Lin PS, Arthur DW. Three-dimensional conformal external beam radiotherapy (3D-CRT) for accelerated partial breast irradiation (APBI): what is the correct prescription dose? Am J Clin Oncol. 2006; 29:474–478.

29. Joosten A, Matzinger O, Jeanneret-Sozzi W, Bochud F, Moeckli R. Evaluation of organ-specific peripheral doses after 2-dimensional, 3-dimensional and hybrid intensity modulated radiation therapy for breast cancer based on Monte Carlo and convolution/superposition algorithms: implications for secondary cancer risk assessment. Radiother Oncol. 2013; 106:33–41.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download