INTRODUCTION
Breast cancer is the most common cancer in women worldwide and the leading cause of cancer death among women. This malignancy constitutes 33% of cancer among women and is responsible for 19% of cancer-related deaths [
1]. Breast cancer incidence and mortality rates vary in different parts of the world, with the highest incidence rate in the United States and the lowest in Asian countries. However, the incidence is increasing in Asian countries [
2]. According to statistics provided by the American Cancer Society in 2013, the incidence of breast cancer is 1 in every 8 women in the United States and the associated death rate is 1 in 36; it was estimated that 232,340 new cases of breast cancer would be diagnosed in the United States in 2013 [
3]. In Iran, according to the National Center for Cancer Registration, breast cancer incidence has increased dramatically from 2001 considering breast cancer indices; in 2010, 23% of all cancers diagnosed in women were breast cancer cases. The incidence rate of breast cancer is estimated to be 22.09% in 100,000 women, with the age-standardized incidence rate estimated as 28.25% in 100,000 women [
2]. Moreover, the distribution of breast cancer incidence in different age groups varies between Iranian and Western women, and studies have indicated that the mean age of patients in Iran is approximately 10 years younger than in Western countries [
4]. Tabriz is the capital of East Azerbaijan Province, with a population of over 1.5 million people, and is the largest, most populous, and industrialized city in the North West of Iran; according to recent reports from the National Center for Cancer Registration, the incidence of breast cancer is higher in East Azerbaijan than in neighboring provinces [
2].
The etiology of breast cancer is still poorly understood and known risk factors for breast cancer explain only a small proportion of cases. Numerous epidemiological studies conducted over the last 3 decades in different populations have identified a spectrum of well-established and probable risk factors for breast cancer. In addition to its association with genetic and reproductive factors, breast cancer risk displays wide ethnic and geographic variation [
3,
5,
6]. Considering the geographic variation in breast cancer risk, the increased incidence of breast cancer in developing countries such as Iran, and the limited studies on the risk factors for breast cancer, especially in East Azerbaijan Province, the present study aimed to clarify the risk factors for breast cancer in this region; these included socioeconomic status (educational level and income), obesity, smoking, stress, migration, marital status, age at menarche, number of pregnancies, age at first pregnancy, breastfeeding, abortion, infertility, and menopausal status. Most of the previous studies in Iran have investigated reproductive factors, adverse events, or nutritional factors separately [
7,
8,
9]; the present study simultaneously assessed a greater number of probable risk factors (reproductive and lifestyle) to form a comprehensive investigation. By helping to identify risk factors, especially in the East Azerbaijan Province of Iran, the results can then guide the creation of new prevention strategies.
METHODS
This hospital-based case-control study was conducted on 420 women (140 cases and 280 controls) in the Imam Reza Teaching Hospital of Tabriz, between December 2012 and September 2013. The study sample size was determined by considering the 95% confidence interval (CI), a power of 80%, the prevalence of oral contraceptive use (59% in the case group and 49% in the control group) [
7], and an odds ratio (OR) of 2 (where α=0.05, 1-β=0.80) according to the method given by Rothman and Greenland, 1998 [
10]:
The case group was selected from patients who had been referred to the Oncology Clinic of the Imam Reza Hospital, the only public oncology hospital in Tabriz, for chemotherapy, radiation therapy, or follow-up. They had been diagnosed via histopathological analysis as having breast cancer during the previous year. For each case, two age-matched (group-matched) controls were selected from orthopedic, surgery, ear, nose, and throat, and trauma clinics of the Imam Reza College Hospital of Tabriz, who did not have neoplastic or hormonal disease. A history of hysterectomy was considered an exclusion criterion in both the case and control groups.
Ethical approval for this study was obtained from the Ethics Committee of the Research Centre of Hematology and Oncology of Tabriz University of Medical Sciences, with the reference number 5/4/81. Data were collected through face-to-face interviews by trained female interviewers after obtaining the verbal consent of participants, who were also assured of confidentiality and their right to withdraw without prejudice. A questionnaire, designed after reviewing relevant books and articles, was used to complete the data; 10 professors and experts in this area made corrections to the questionnaire. The questionnaire included 11 questions on the following demographic characteristics: age, socioeconomic status (educational level and income), marital status, residence, and occupation. A further 22 questions were about the following general risk factors: smoking, use of alcohol, hookahs, and drugs, a family history of breast cancer, physical activity (at least 30 minutes, 3 times a week), exposure to severe stress during the last 5 years (bereavement, divorce, bankruptcy, and loss of employment being the major stressors), migration, anthropometry (weight and height measurements for the calculation of body mass index [BMI]), and diet (containing sufficient fruit and vegetables, or high fat). Finally, the questionnaire included 22 questions on menstrual and reproductive history including the marital status, parity, age at first pregnancy, age at menarche, menopausal status, breastfeeding (history and duration), oral contraceptive pill (OCP) use (history and duration), infertility, abortion, benign lumps, breast biopsy, and hormone therapy. We defined "sufficient fruit and vegetables" as at least five servings of fruit and vegetables per day (1 serving of fruit is equal to 1 medium apple, orange, or banana, or three-quarters of a cup of strawberries or fruit juice; 1 serving of vegetables is equal to half a cup of cooked vegetables or 1 cup of raw vegetables, including cabbage, lettuce, carrots, zucchini, or eggplant, or a medium-sized potato or tomato). We defined a high-fat diet as the daily consumption of fried food, animal fat, mayonnaise and high-fat dairy produce.
Self-reported data (use of alcohol, cigarettes, hookahs, and drugs, abortion, and a history of benign lumps) were verified against corresponding entries in medical records, because of concerns about possible under-reporting, and any discrepancy rectified. The height and weight of women in the case and control groups were measured in the clinic and the BMI calculated as weight in kilograms divided by height in meters squared.
The collected data were analyzed using the SPSS software version 18 (SPSS Inc., Chicago, USA); values of the mean, standard deviation, and OR with 95% CI were calculated. Univariate logistic regression analysis was performed to calculate ORs and to examine the predictive effect of each factor on breast cancer risk; p<0.05 was considered statistically significant. Factors significantly associated with breast cancer risk as well as borderline variables (p<0.1) in the univariate analysis were entered into stepwise multivariate logistic models.
RESULTS
In this study, 420 women were included, 140 in the case group and 280 in the control group. The mean age of cases was 47.6±10.7 years and that of controls 46.8±10.4 years (p=0.18); 61.9% of women lived in urban areas and 39.1% lived in rural areas.
Table 1 shows the distribution of variables, ORs, and 95% CIs from the univariate regression analysis of general risk factors, while
Table 2 shows the corresponding data for reproductive risk factors. In the present study, there was no significant relationship of marital status, breast cancer in a second-degree relative, physical activity, smoking, BMI, parity, infertility, or hormone therapy after menopause with breast cancer. Considering general risk factors, there was a significant relationship of educational level (OR, 2.55; 95% CI, 1.42-4.57), breast cancer in a first-degree relative (OR, 2.17; 95% CI, 1.24-4.17), a high-fat diet (OR, 3.01; 95% CI, 1.97-4.06), passive smoking (OR, 2.02; 95% CI, 1.31-3.12), migration (OR, 3.8; 95% CI, 2.1-6.87), stress (OR, 5.19; 95% CI, 3.33-8.09), income (OR, 2.17; 95% CI, 1.33-3.54), and a diet containing sufficient fruit and vegetables (OR, 0.37; 95% CI, 0.24-0.57) with breast cancer (
Table 1). It should be noted that, as alcohol, drug and hookah use were recorded in a very low number of women (less than 1% in both case and control groups), these variables were not entered in statistical analyses. Among menstrual and reproductive factors, age at menarche (OR, 2.5; 95% CI, 1.34-4.67), number of pregnancies (OR, 2.84; 95% CI, 1.38-5.84), age at first pregnancy (OR, 3.48; 95% CI, 1.41-8.61), abortion (OR, 1.69; 95% CI, 1.12-2.56), and OCP use (OR, 3.59; 95% CI, 2.33-5.53) showed a significant association with breast cancer (
Table 2).
Table 3 shows the results of the multivariate logistic regression analysis of risk factors for breast cancer. It should be noted that borderline variables (
p<0.1), such as menopausal status, breastfeeding and a history of benign breast lumps, were entered into the multivariate logistic regression model along with other significant variables. Educational level (OR, 4.78; 95% CI, 2.11-10.38), menopausal status (OR, 2.54; 95% CI, 1.41-4.59), a high-fat diet (OR, 2.76; 95% CI, 1.51-5.04), abortion (OR, 2.13; 95% CI, 1.20-3.79), passive smoking (OR, 2.76; 95% CI, 1.51-5.04), OCP use (OR, 3.18; 95% CI, 1.80-5.59), stress (OR, 3.05; 95% CI, 1.74-5.36), and migration (OR, 3.09; 95% CI, 1.39-6.90) showed a statistically significant association with breast cancer in the multivariate logistic regression model; therefore, they were considered to be risk factors. Breastfeeding (OR, 0.39; 95% CI, 0.16-0.97) and a diet containing sufficient fruit and vegetables (OR, 0.22; 95% CI, 0.12-0.39) both showed a statistically significant association with breast cancer, but they were negatively associated with breast cancer risk and, therefore, protective factors.
DISCUSSION
The present study shows that educational level, menopause, a high-fat diet, abortion, passive smoking, OCP use, stress, and migration are risk factors for breast cancer in Tabriz; breastfeeding and an adequate intake of fruit and vegetables were protective against breast cancer.
The mean age of patients with breast cancer in this study was 47.6±10.7 years, which is consistent with the findings of previous studies in Iran and confirms a young age for breast cancer development in Iranian women [
7].
With regard to socioeconomic status (educational level and income) and its association with breast cancer, some studies have suggested that educational level is associated with an increased breast cancer risk [
11,
12]. In our study, the results of univariate regression analysis revealed a significant relationship between the variables of socioeconomic status and breast cancer. However, in the final regression model, income showed no significant association with breast cancer, whereas with an increase in the level of education, the breast cancer risk also increased, though secondary-level rather than university-level education was associated with the higher breast cancer risk. A possible explanation for this might be the low number of individuals with a university education in this study. It appears that the association of the socioeconomic status with breast cancer risk is mostly due to other factors; socioeconomic status can affect reproductive behavior, for example, the number of children, age at first birth, childlessness, and the duration of breastfeeding as well as lifestyle factors, including physical activity and diet [
12].
Some studies have suggested an association of dietary factors with breast cancer [
13,
14]. Fruit and vegetables are rich sources of carotenoids and flavonoids, which are potentially protective against cancer [
13]. The results of our study show that eating an amount of fruit and vegetables equivalent to at least 5 units per day reduces the risk of breast cancer. Findings reported by Boggs et al. [
14] from a study in Boston, United States showed that total vegetable consumption was associated with a decreased risk of breast cancer. Some studies have shown an association between the consumption of high-fat food and breast cancer [
15,
16]; the findings of the present study are consistent with the published reports. In the present study, the daily intake of fat was not assessed but an evaluation of fat intake was made through questions regarding the consumption of fatty food, such as fried food, high-fat dairy products, and mayonnaise, and the predominant method of cooking in the home. It appears that further research is needed on this issue.
The present study showed that exposure to severe stress during the preceding 5 years can increase the risk of breast cancer, with bereavement, divorce, bankruptcy and loss of employment being the major stressors; this is consistent with the report by Lillberg et al. [
17] after performing a study in Finland, which showed that divorce/separation, death of a husband, and death of a close relative or friend were each associated with an increased risk of cancer. It appears that examining the impact of adverse events alone is insufficient and even misleading, but when it is associated with mechanisms of adaptation and social support, it can reveal the real impact of stress on disease. Therefore, it seems that more research on this issue, simultaneously examining social support and coping mechanisms, is needed.
The findings of various studies on the relationship of smoking and breast cancer risk are controversial, but some studies have suggested an association [
18,
19]. In the present study, no relationship between smoking and breast cancer was observed. A report of a study in Serbia proposed smoking as a risk factor for breast cancer and showed that people who quit smoking when over 50 years of age were at a higher risk [
19]. The present study shows that passive smoking has a significant association with breast cancer; similar results were reported for a study in the United States by Petralia et al. [
20]. Culturally, there is a low prevalence of smoking among Iranian women, and owing to this, smoking by family members and partners was considered as a possible risk factor for breast cancer in the present study; however, there is still a need for further investigation.
Studies of immigrants provide important information on the contribution of environmental and genetic factors in the etiology of various cancers. Some studies have suggested an association of a geographic area and of the living environment with breast cancer [
21,
22]. In the present study, relocation and migration from a rural to urban area has been identified as a risk factor for breast cancer; this may provide strong evidence for the role of the living environment and lifestyle in cancer risk [
21].
The present study did not show any significant relationship between physical activity and breast cancer. In a review article by Friedenreich [
23], no association between physical activity and breast cancer was found for premenopausal women, but in postmenopausal women, physical activity throughout the lifetime reduced the risk of breast cancer; in the present study, we did not examine premenstrual and postmenopausal women separately. The differences between our findings and those reviewed by Friedenreich might also be owing to the different definitions of physical activity. In the present study, the variable of exercise was defined as physical activity of at least 30 minutes duration, 3 times a week, whereas in the Friedenreich review, they examined all forms of physical activity (occupational, household, and recreational), throughout the lifetime, and assessed all three parameters of physical activity (frequency, intensity, and duration).
Our study showed no significant relationship between BMI and breast cancer, consistent with the study conducted by Hadjisavvas et al. [
6] in Cyprus. One possible reason for the lack of a significant relationship in the present study is the measuring of BMI when a patient is already suffering from breast cancer; future studies should consider this matter.
Many studies have suggested an association of breast cancer risk with menstrual and reproductive factors. Some previous studies suggested that induced or spontaneous abortion were associated with breast cancer [
5,
24] but not all of them [
25]. The results of the present study show that abortion increases breast cancer risk, although spontaneous and induced abortions were not separated in our analyses; future studies could examine each type of abortion individually.
The findings of our study indicate that OCP use is a risk factor for breast cancer; this is consistent with some previous studies [
8,
26]. In our study, the potential to investigate different types of OCP (combinations of estrogen and/or progestin) and the duration of usage, thereby providing results that were more accurate, was not achieved; more studies should be carried out considering different OCP combinations.
The majority of reported studies have shown an association between breastfeeding and the risk of breast cancer [
5,
6,
27]. The present study shows that women with a history of breastfeeding have a lower risk of breast cancer; the results showed that with the increase in the duration of breastfeeding, the risk of breast cancer reduced. Consistent with this, in a collaborative reanalysis of 47 studies, the authors also concluded that women with a history of breastfeeding had a lower risk of breast cancer, and that a prolonged duration of lactation further reduced the risk [
27]. Thus, the majority of studies have mentioned breastfeeding as a protective factor against breast cancer. Breastfeeding is hypothesized to primarily reduce the risk of breast cancer through two mechanisms, i.e., differentiation of breast tissue and reduction of the number of ovulatory cycles in a lifetime [
5].
In some studies, early menarche has been introduced as a risk factor for breast cancer [
6,
28]. This is consistent with the hypothesis that breast cancer risk is related to the extent of mitotic activity in the breast. This mitotic activity is driven by estrogen and progesterone exposure during the luteal phase of the menstrual cycle, which in turn determines the probability of tumorigenic somatic events; early menarche increases these mitotically active periods in the breast and consequently increases the risk of breast cancer [
6]. In the present study, menarche under the age of 12 years was significant in the univariate regression analysis but in the final model, it did not show a significant relationship with breast cancer.
With regard to the number of children a woman bears, the present study used univariate regression analysis to show that with an increasing number of children, the risk of breast cancer decreased; however, there was no significant relationship between the number of children and breast cancer risk in the final model. Some studies have found that increasing parity decreases breast cancer risk in both premenopausal and postmenopausal women [
6,
29]. The present study did not show a statistically significant association of late pregnancy with breast cancer in the final model; this is consistent with the report by Hadjisavvas et al. [
6], wherein only a weak association was observed between late pregnancy and breast cancer.
Surprisingly, the association between a family history of breast cancer and breast cancer risk in the present study was not significant in the multivariate model, although it was significant in the univariate model. Our findings show that most of the risk factors for breast cancer in the Tabriz region of Iran are related to lifestyle. It appears that the Iranian diet and lifestyle have changed during the process of rapid westernization. Generally, the increasing adaptation to a Western lifestyle is an important determinant of the increasing breast cancer incidence in developing countries.
Our study has some limitations; because of its case-control nature, selection bias and recall bias arise in the study. Recall bias means that when the subjects from case and control groups are asked about factors and events related to a disease, it is more likely for the case group to remember these than the control group. In our study, most of the data were obtained from the women's self-reports, so recall bias was more probable, therefore, medical records were checked to confirm the history of participants. In addition, women referred to the hospital were used in the control group; this method of selection can help somewhat in minimizing selection and recall bias.
In summary, in the present study, lifestyle factors appear to pose a greater risk than the effects of reproductive factors for breast cancer of women in Tabriz. Therefore, given that certain dietary factors and stress were identified as risk factors in this study, lifestyle changes as well as providing necessary training in the field are now required to promote a healthy diet, a change of unhealthy eating patterns, and methods for coping with stress.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


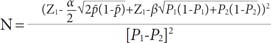
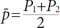

 XML Download
XML Download