Abstract
Fibroadenoma is the most common breast tumor in women. Malignant transformation occurs rarely within fibroadenoma at older ages. Clinicians, radiologists, and pathologists need to be aware of malignant transformation within fibroadenomas. Radiologic studies play an important role in the diagnosis of fibroadenoma; however, radiologic findings are often nonspecific for malignancy and may appear completely benign. We detected an occult ductal carcinoma in situ that originated inside a fibroadenoma by using shear wave elastography. We report shear wave elastography findings of ductal carcinoma in situ within fibroadenoma and discuss the diagnostic role of this modality.
Fibroadenoma is the most common breast tumor in women and is characterized by proliferation of both epithelial and stromal components of the terminal ductal unit. Women can present with fibroadenoma at any age, but the peak incidence is in the second and third decades of life [1]. Furthermore, fibroadenomas are common incidental findings in patients undergoing radiological screening [2,3]. Fibroadenomas expand in the presence of high estrogen and progesterone levels, particularly with pregnancy and lactation. In the postmenopausal period, they undergo atrophic changes, which manifest as a "shrinking" growth habit. Malignant transformation occurs rarely within fibroadenomas [4,5,6]. Nevertheless, radiologic findings are often nonspecific for malignancy and may appear completely normal. The development of new techniques to reveal these malign transformations is needed. Shear wave elastography (SWE) is a novel ultrasonographic technique that provides elasticity information about soft-tissue components. We report a case of ductal carcinoma in situ (DCIS) that originated within a fibroadenoma and was diagnosed histopathologically after suspicious findings were detected on SWE. To our knowledge, this is the first case in the literature to date of DCIS within fibroadenoma that was diagnosed using SWE.
A 30-year-old woman who had undergone monitoring of multiple fibroadenomas in both breasts for years presented at our clinic. There was no own/family history of breast or ovarian cancer, and the patient had regular menstrual cycles. Physical examination revealed an elastic, firm, well-defined retroareolar mass in the left breast measuring approximately 3 cm. Axillary and supraclavicular lymph nodes were not palpable. Ultrasound (US) examination revealed a macrolobulated and well-defined heterogeneously hypoechoic solid retroareolar mass with sparse microcalcifications and measuring 33×12 mm parallel to the cutaneous surface in the left breast; the mass had enlarged slightly since the previous examination (Figure 1A). SWE was applied with the Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France), which was equipped with a 4- to 15-MHz linear-array transducer, to investigate mass stiffness of the lesion quantitatively. The maximum elasticity value of the mass, measured in a 2 mm diameter region of interest, was 106.1 kPa in the lateral portion (far from the nipple) and 21.8 kPa in the medial portion (close to the nipple) (Figure 1B). The lateral portion showed significantly increased stiffness values compared with the medial portion of the mass. The other breast masses did not show any significant SWE findings. Doppler US showed no increase in vascularity. Mammography examination was performed because of the presence of microcalcifications and revealed sparse uniform microcalcifications within the mass. US-guided 14G core needle biopsy was performed. Considering the SWE findings, we aimed to obtain the biopsy sample from the lateral portion of the mass. Samples were also obtained from the medial portion for comparative purposes. Pathological examination revealed high-grade DCIS with comedonecrosis within the lateral portion of the fibroadenoma.
Breast magnetic resonance imaging (MRI) was ordered to evaluate the extent of the mass and to rule out any suspicious concomitant lesions before surgery. Contrast (gadolinium diethylenetriaminepentaacetic acid) enhanced breast MRI with routine sequences revealed two other breast masses, one in each breast, with benign features compatible with sonographic findings in addition to the abovementioned mass lesion. In the retroareolar area of the left breast, there was a bilobulated mass with clearly defined contours (Figure 2A). In T1-weighted axial scans, both lateral and medial portions were hypointense, but in T2-weighted axial scans, the medial portion was heterogeneously hyperintense while the lateral portion was hypointense. Contrast-enhanced images showed significant early, rapid contrast enhancement followed by slow, late-phase washout kinetics (type III) in the lateral portion, and slow and minimally persistent enhancement kinetics (type I) in the medial portion (Figure 2B and C). After completion of radiological and pathological examinations, the patient underwent breast-conserving surgery. Postoperative specimen evaluation confirmed the diagnosis of high-grade DCIS arising within the fibroadenoma and mild epithelial hyperplasia. Serial sections revealed DCIS areas with malignant pleomorphic ductal cells within fibroadenomatous stroma surrounded by basal membrane. On immunohistochemical examination, myoepithelial cells in these areas showed positive staining for smooth-muscle actin (Figure 3).
Fibroadenoma of the breast is a common benign tumor of young women. Although it is such a common benign lesion, it may show malign degeneration. The prevalence of carcinoma within fibroadenoma in screened populations is reported to be 0.02% to 0.3% [2,3]. Carcinoma within fibroadenoma is predominantly found in women over 40 years of age [7]. In contrast, the patient in our report was 30 years of age and malignancy was unexpected.
US is an important modality for the detection, characterization, and follow-up of fibroadenomas meeting the Breast Imaging Reporting and Data System (BI-RADS) criteria. There are no strict criteria for the determination of the presence of malignancy in BI-RADS 2 or 3 lesions, but newly developed border irregularity, heterogeneous internal structure, and increase in size are suspicious sonographic findings justifying tissue sampling in our institution. Operators should carefully search for morphological changes precursoring focal in situ transformations in these common benign lesions. Despite technological developments in grayscale sonography, the salient abnormality for suspicion of malignancy is still increase in size. However, follow-up assessments vary among operators in sonographic examination, and size estimation is not always a reliable follow-up method because of this operator variability issue. The technical capability of the sonography device for adequate visualization is also crucial.
Opinions differ regarding evaluation of the malignancy probability with Doppler sonography studies. It is reported that malignant lesions tend to be hypervascularized because of neo-angiogenesis and demonstrate hypervascularity (92.9%), irregular vessels (73.2%), and rich vascularization (vessel/mass ratio >10% in 54.2% of cases), as well as more than one vascular pole [8]. Furthermore, positive signals on color power Doppler sonography were found in 22 of 32 patients (69%) with DCIS [9]. However, flow visualization on power Doppler sonography indicates a higher possibility of malignancy but is not useful as the main sign for malignancy [10]. Doppler studies are used to detect increased vascularization due to neo-angiogenesis. However, tumor angiogenesis was not detected in 75% of in situ cancers [11]. With regard to the tumor type in our case, Doppler sonography has been reported to be useful for detection of in situ cancer transformation within fibroadenoma [12] but did not provide sufficient information in our patient.
Because there are no definite clinical or radiological criteria for diagnosing carcinoma within fibroadenoma, percutaneous biopsy should be performed as the first-line guidance modality for histopathological examination to rule out malignancy in cases of suspected nonsimple fibroadenoma. New technological advances are needed for predicting malignant transformation.
SWE is a novel technique for obtaining elastograms of soft tissues, and SWE elasticity values are helpful in differentiating benign from malignant breast masses [13]. The average mean elasticity values of DCIS (117.8±54.72 kPa) were higher than those of fibroadenoma (49.58±43.51 kPa) in the study by Chang et al. [14]. According to Evans et al. [15], fibroadenomas on SWE imaging showed low mean stiffness (average, 28 kPa; range, 18 to 44 kPa); however, the mean stiffness value of DCIS was 76 kPa. Correspondingly, the mean value of the lateral portion of the mass in our case was 77 kPa while that of the medial portion, which did not include malignant transformation, was 12 kPa on SWE imaging. SWE imaging overcomes one of the major drawbacks encountered in elastography-operator dependency. The technique does not use pressure application and manual tracing of the lesion. Quantitative data is acquired and the need for operator experience is minimal.
In our case, we performed US-guided core needle biopsy intentionally of the lateral portion of the mass, which showed higher stiffness values than the medial portion on SWE imaging, and SWE findings correlated well with pathological results. There are no reliable methods in US and Doppler examinations to obtain quantitative results in a malignant transforming mass. On the other hand, SWE yields quantitative results, so this imaging method might be useful to pinpoint malignant areas before performing biopsy. Our experience suggests that SWE may be an efficient supplemental tool for routine fibroadenoma follow-up to detect suspicious areas by measuring and comparing quantitative stiffness values of the lesions.
Figures and Tables
Figure 1
Ductal carcinoma in situ within a fibroadenoma in a 30-year-old female. (A) In the gray scale ultrasonography image, there is a bilobulated lesion parallel to the skin surface. Similar heterogenously hypoechoic appearence is seen in the both compound. A few hyperechoic microcalcification are seen also. (B) In the elastography image, 2 mm diameter region of interest calculates maximum elasticity value of lateral portion of mass as 106.1 kPa, on the other hand it was measured 21.8 kPa on medial portion. Note that display was saturated no shear wave elastography artefact was present.
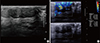
Figure 2
Contrast-enhanced magnetic resonance imaging (MRI) of the breast. (A) Breast MRI examination findings of the lesion. Contrast enhanced T1W oblique reformat image shows significantly enhanced lateral portion (arrow) despite the medial portion (arrowhead) of the lesion. (B) Color coding axial image depicts the lesion clearly. (C) Dynamic evaluation is revealed as early intermediate contrast enhancement followed by late phase washout kinetics (type III) in the lateral portion while slow and minimal persistant enhancement kinetics (type I) in the medial portion (not shown).

Figure 3
Microscopic findings of ductal carcinoma in situ (DCIS) within fibroadenoma. (A) Magnification view shows DCIS and mild epithelial hyperplasia (arrows) in the fibroadenoma which corresponds to the shear wave elastography and magnetic resonance imaging findings (not all parts of the DCIS portion could be shown due to different slice course) (H&E stain, ×20). (B) Pleomorfic malignant cells are seen within an eosinophilic boundary of basement membran (H&E stain, ×200). (C) This demonstrates the myoepithelial layer around the well defined island of malignant cells with smooth-muscle actin (SMA) immunohistochemistry (×200).

References
1. Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med. 1998; 13:640–645.

2. Deschênes L, Jacob S, Fabia J, Christen A. Beware of breast fibroadenomas in middle-aged women. Can J Surg. 1985; 28:372–374.
3. Ozzello L, Gump FE. The management of patients with carcinomas in fibroadenomatous tumors of the breast. Surg Gynecol Obstet. 1985; 160:99–104.
4. Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast: a clinicopathologic study of 105 patients. Am J Clin Pathol. 1991; 95:614–622.

5. Abu-Rahmeh Z, Nseir W, Naroditzky I. Invasive ductal carcinoma within fibroadenoma and lung metastases. Int J Gen Med. 2012; 5:19–21.

6. Abe H, Hanasawa K, Naitoh H, Endo Y, Tani T, Kushima R. Invasive ductal carcinoma within a fibroadenoma of the breast. Int J Clin Oncol. 2004; 9:334–338.

7. Kuijper A, Preisler-Adams SS, Rahusen FD, Gille JJ, van der Wall E, van Diest PJ. Multiple fibroadenomas harbouring carcinoma in situ in a woman with a family history of breast/ovarian cancer. J Clin Pathol. 2002; 55:795–797.

8. Giuseppetti GM, Baldassarre S, Marconi E. Color Doppler sonography. Eur J Radiol. 1998; 27:Suppl 2. S254–S258.

9. Yang WT, Tse GM. Sonographic, mammographic, and histopathologic correlation of symptomatic ductal carcinoma in situ. AJR Am J Roentgenol. 2004; 182:101–110.

10. del Cura JL, Elizagaray E, Zabala R, Legórburu A, Grande D. The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. AJR Am J Roentgenol. 2005; 184:1788–1794.

11. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8.

12. Tiu CM, Chou YH, Chiou SY, Hsu CY, Chen SP, Chiang HR, et al. Development of a carcinoma in situ in a fibroadenoma: color Doppler sonographic demonstration. J Ultrasound Med. 2006; 25:1335–1338.
13. Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. Breast lesions: quantitative elastography with supersonic shear imaging: preliminary results. Radiology. 2010; 256:297–303.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download