Abstract
Purpose
Photodynamic therapy (PDT) is gaining increasing recognition for breast cancer treatment because it offers local selectivity and reduced toxic side effects compared to radiotherapy and chemotherapy. In PDT, photosensitizer drugs are loaded in different nanomaterials and used in combination with light exposure. However, the most representative issue with PDT is the difficulty of nanomaterials to encapsulate anticancer drugs at high doses, which results in low efficacy of the PDT treatment. Here, we proposed the development of the poly(N-isopropylacrylamide) (PNIPAM) microgel for the encapsulation of methylene blue, an anticancer drug, for its use as breast cancer treatment in MCF-7 cell line.
Methods
We developed biocompatible microgels based on nonfunctionalized PNIPAM and its corresponding anionically functionalized PNIPAM and polyacrylic acid (PNIPAM-co-PAA) microgel. Methylene blue was used as the photosensitizer drug because of its ability to generate toxic reactive oxygen species upon exposure to light at 664 nm. Core PNIPAM and core/shell PNIPAM-co-PAA microgels were synthesized and characterized using ultraviolet-visible spectroscopy and dynamic light scattering. The effect of methylene blue was evaluated using the MCF-7 cell line.
Results
Loading of methylene blue in core PNIPAM microgel was higher than that in the core/shell PNIPAM-co-PAA microgel, indicating that electrostatic interactions did not play an important role in loading a cationic drug. This behavior is probably due to the skin layer inhibiting the high uptake of drugs in the PNIPAM-co-PAA microgel. Core PNIPAM microgel effectively retained the cationic drug (i.e., methylene blue) for several hours compared to core/shell PNIPAM-co-PAA and enhanced its photodynamic efficacy in vitro more than that of free methylene blue.
Breast cancer is the most commonly diagnosed cancer among women worldwide, with more than 1.3 million individuals diagnosed each year [1,2]. There are various approaches to inhibit the growth of breast cancer cells. Among these, photodynamic therapy (PDT) is gaining increasing recognition and is used for the treatment of various types of cancers [3]. PDT causes low systemic toxicity and can be locally applied onto a specific region by selectively illuminating the tumor by light, while leaving normal tissues untouched, thus offering much lower toxic side effects than those induced by radiotherapy and chemotherapy. In addition, PDT can be applied alone or in combination with other treatment methods such as surgery [4]. In PDT, photosensitizers are excited by harmless visible light of a defined wavelength, which excites the photosensitizer drugs to their high-energy triplet state. The excited photosensitizers react with cellular oxygen to form toxic reactive oxygen species (ROS) such as singlet oxygen and oxygen radicals. These ROS inhibit the growth of the breast cancer cells by inducing apoptosis, necrosis, and/or autophagy [4,5,6].
Many kinds of photosensitizers have been developed so far for treating breast cancer. Among them, methylene blue is very popular because it is highly photo-stable, easily eliminated from the body, and has minimum toxicity [7]. Hence, methylene blue has also been approved as a potential PDT drug for the local treatment of periodontal disease because of its relative low toxicity and high generation yield of singlet oxygen [8,9,10]. However, methylene blue also has some drawbacks, because its activity decreases in vivo. Methylene blue is converted to its nonphotosensitizer leuco-isomer, which shows negligible photodynamic activity in biological environments [11]. To improve the performance of methylene blue, nanoparticles containing photosensitizing agents have been used. In fact, nanoparticles encapsulating photosensitizers generate high volumes of reactive oxygen, which kills cancer cells [12] and enhances the local distribution of photosensitizers near the cancer, thus reducing immunogenicity as well as other side effects [13].
Biodegradable polymeric nanoparticles are popular nanomaterials because their size and morphology can be easily tuned to encapsulate and stabilize PDT drugs [14,15,16]. In this context, poly(N-isopropylacrylamide) (PNIPAM) microgel proved promising for photodynamic drug delivery because the microgel is physically and mechanically similar to the extracellular matrix of cells, making is particularly favorable for use in the body [17]. PNIPAM microgel protects the active form of photosensitizers from enzymatic or environmental degradation in vivo and provides porous microstructures, which facilitate the diffusion of molecular oxygen to inhibit the growth of cancer cells during PDT [18,19]. PNIPAM microgels are temperature sensitive [20] and interact with water via hydrogen bonding below 32℃, which is the lower critical solution temperature (LCST). Above LCST, hydrophilic PNIPAM becomes hydrophobic and releases water. This behavior facilitates the loading of hydrophilic drug at room temperature, at which the microgel is swollen, thus optimizing drug entrapment into the gel phase because of optimal drug diffusion. Subsequently, when placed at physiological temperature, the microgel collapses; this reduces the effective diffusion coefficient of drugs through the microgel phase and maintains the sustained release of drugs.
We have synthesized different kinds of PNIPAM microgels and characterized their drug loading ability to enhance the efficiency of PDT. Here, we have employed PNIPAM microgels as well as functionalized anionic core-shell microgels consisting of PNIPAM and poly acrylic acid (PNIPAM-co-PAA) to encapsulate methylene blue. PDT was performed and the cell viability of the MCF-7 breast cancer cell line was observed.
PNIPAM core microgel particles were prepared via free radical polymerization as reported previously. Polymerization was performed in a 500-mL reaction vessel equipped with a mechanical stirrer, reflux condenser, thermometer, and N2 gas inlet. N-isopropylacrylamide (NIPAM, 0.5665 g, 88.7%; Sigma-Aldrich Inc., St. Louis, USA), N,N'-methylene-bis-(acrylamide [BIS], 0.0193 g, 3.0%; Sigma-Aldrich Inc.), and sodium dodecyl sulfate (SDS, 0.0242 g, 3.8%; Sigma-Aldrich Inc.) were dissolved in 74 mL of water and the solution was heated to 70℃ while being purged with N2 gas and stirred vigorously for approximately 1 hour. After 1 hour, polymerization was initiated by adding 0.0288 g (4.5%) of ammonium persulfate (APS; Sigma-Aldrich Inc.) and reacting for 4 hours under N2 stream. The particles were purified by centrifuging the solution, decanting the supernatant solution, and resuspending it in deionized water. This process was repeated four times.
The first batch of microgel particles (core) served as nuclei for the second stage of polymerization for shell addition. In the shell addition step, 5 mL of crude microgel core and SDS (0.0007 g) were dissolved in 15 mL of water and heated to 70℃ while stirring under N2 gas stream. NIPAM (0.048 g) and BIS (0.0017 g) were dissolved in 5 mL of water degassed under N2 and added to the crude microgel core solution. Finally, acrylic acid (AAc; Sigma-Aldrich Inc.) solution (0.003 and 15 mL) was added. This solution was kept at 70℃ under a stream of N2. Polymerization was then initiated by the addition of 0.0009 g of APS dissolved in 1 mL of water and carried out for 5 hours. All core/shell particles were then purified by centrifuging the solution, decanting the supernatant, and resuspending in fresh water. This process was repeated several times.
The hydrodynamic radius of both core and core/shell microgels was determined using Dynamic light scattering. The suspensions of microgel were held at different temperatures for 5 minutes to achieve thermal equilibrium before measuring. The hydrodynamic radius was calculated by using the Stokes-Einstein equation:
where, KB is the Boltzmann's constant, T is the absolute temperature, η is the viscosity, r is the radius, and D is the diffusion constant [21].
The size of the PNIPAM core microgel was measured at pH 7 at various temperatures (25℃-40℃), while the size of the PNIPAM-co-PAA core/shell microgel was measured at various temperatures (25℃-40℃) at pH 3 and 7.4.
Before the addition of methylene blue to the microgel, a calibration curve was obtained by using known concentrations of methylene blue (Fisher Scientific, Fair Lawn, USA) in phosphate buffer. The absorbance of methylene blue was recorded at 664 nm by using a Cary 100 ultraviolet-visible (UV-vis) spectrometer (Agilent Inc., Foster City, USA). The UV-vis spectra and calibration curve showed a maximum concentration of methylene blue of 14.97 µM (data not shown). The fabricated core and core/shell microgels were mixed with an equal volume of 10 mM phosphate buffer (5 mL). Then, 0.5 mL of core or core/shell microgel was mixed with 0.457 mL of 550 µM methylene blue. The final concentration of drug was kept at 85 µM by adding 2 mL of 10 mM phosphate buffer. Then, the microgel containing methylene blue was incubated for 24 hours. After 24 hours, the solution of microgel with loaded methylene blue was centrifuged for 2 hours. Finally, the absorbance of the supernatant was measured at 664 nm. The percentage of methylene blue loaded into the microgel was calculated from the following equation:
The microgels containing the loaded methylene blue at pH 7.4 were dropped separately in a CoverWell perfusion chamber gasket (Invitrogen Corp., Eugene, USA). After being completely dried up in air, the chamber gasket was placed vertically on the side of a 1-mL cuvette containing 1 mL of phosphate buffer (10 mM, pH 7.4). Drugs release experiments were conducted at 25℃ and 37℃ at pH 7.4. For the core/shell microgel, drug release experiments were conducted at pH 3 by keeping the temperature at 37℃. The cuvette was placed such that the incident light from the UV-vis spectrometer did not hit the microgel/drug film. The solution was constantly stirred to maintain homogeneity. At preselected intervals, the absorbance of the sample was measured at 664 nm. The percentage of methylene blue released was calculated as follows:
Control experiments using only red light or blank microgel (no methylene blue) under similar conditions have been conducted and the results compared to those from free methylene blue solution and methylene blue-encapsulated microgel.
The MCF-7 cancer breast cell line (HTB-22) was purchased from the American Type Culture Collection (Manassas, USA). MCF-7 were cultured in Eagle's Essential Medium (American Type Culture Collection) containing 0.01 mg/mL bovine insulin and 10% fetal bovine serum at 37℃ and 5% CO2 in a T-25 flask. Then, MCF-7 cells were transferred into 96-well plates and incubated for 24 hours. After the medium was removed, fresh culture medium containing 40 µL of methylene blue-encapsulated microgels were added. Similarly, for control experiments, 40 µL of methylene blue alone in culture medium and 40 µL of microgel alone in culture medium were separately added into the well plates containing MCF-7 cells to a final volume of 235 µL of medium. In both cases, the final concentration of methylene blue was maintained either at 25 µM or 0.6 µM. Afterwards, each cell well was irradiated at various interval times by a laser at 663 nm (Research Electro-Optics Inc., Boulder, USA) located at a distance of 1.5 cm from the 96-wells plate. Then, the plate was incubated for 48 hours at 37℃ in 5% CO2 atmosphere.
To examine the cell viability, 20 µL of ([2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] [WST-8]) was added and incubated for 3 hours at 37℃ in 5% CO2 atmosphere. The cell viability was measured by using a microplate reader (Bio-Rad Laboratories, Philadelphia, USA) irradiated at the wavelength of 450 nm. The cell viability was calculated by using the optical density of the samples.
PNIPAM microgel is constituted by temperature-sensitive particles. Below the LCST, the microgel is swollen, hydrated, and has hydrophilic properties, thus making strong hydrogen bonds with water. Above the LCST, the microgel collapses, dehydrates, and becomes hydrophobic due to the disruption of water-polymer hydrogen bonds. Previous studies reported that the LCST of PNIPAM microgel depends on the ionic strength of the solution [22]. Hence, we measured the size of PNIPAM microgel at various temperatures in 10 mM phosphate buffer as well as in deionized water to observe the LCST. Figure 1 represents the hydrodynamic radius of PNIPAM microgel in 10 mM phosphate buffer and in deionized water at pH 7.4. The results showed that, in the presence of the phosphate buffer, the size of PNIPAM microgel decreases from 174 nm to 56 nm, while the temperature was increased from 25℃ to 40℃. In deionized water, the size of microgel decreases from 180 nm to 59 nm with increasing the temperature from 25℃ to 40℃. From Figure 1, the LCST of PNIPAM microgel was derived to be at 32℃ in phosphate buffer and 34℃ in deionized water.
The effects of the temperature and pH were also investigated in core/shell microgel PNIPAM-co-PAA in 10 mM phosphate buffer. Figure 2 denotes the hydrodynamic radius of core/shell microgel at pH 3.0 and pH 7.4 in phosphate buffer. The size of PNIPAM-co-PAA microgel changes from 141 nm to 65 nm with increasing the temperature at pH 3.0, while at pH 7.4, the size of the core/shell microgel changes from 169 nm to 93 nm. The size of the microgel is higher at high pH than that at low pH. The LCST of core/shell microgel is approximately 34℃ and 31℃ at pH 7.4 and 3, respectively. This indicates that the core/shell microgel is more hydrophilic at high pH, because most of the AAc groups in the PNIPAM-co-PAA microgel become deprotonated.
Nonfunctionalized PNIPAM and functionalized PNIPAM-co-PAA microgels were used for the encapsulation of the cationic methylene blue as drug model in breast cancer treatment. The methylene blue loading was 28.94% in core/shell microgel, whereas it was 37.8% in core microgel.
Figure 3 shows the release of methylene blue from functionalized PNIPAM-co-PAA microgel at 37℃ and 25℃ at pH 7.4. The results indicated that methylene blue is releases slowly at 25℃ in comparison to 37℃. After 6 hours at 25℃, the absorbance of methylene blue reaches 0.038, while at 37℃, it reaches 0.32. This result is reasonable because, at 25℃, core/shell microgels swell and retain the hydrophobic drugs, while at 37℃ the microgels shrink and release the drugs. A similar phenomenon was also observed with PNIPAM microgel. Figure 4 represents the release of methylene blue from nonfunctionalized PNIPAM microgel at 37℃ and 25℃ at pH 7.4. The results showed that, after 5 hours, the absorbance of methylene reaches 0.023 and 0.167 at 25℃ and 37℃, respectively.
Figure 5 represents the comparison of methylene blue release from nonfunctionalized PNIPAM and functionalized PNIPAM-co-PAA microgel at 37℃, and shows that, after 6 hours, the release of methylene blue from functionalized PNIPAM-co-PAA microgel is 74%, while from nonfunctionalized PNIPAM it is 86%.
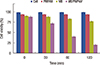
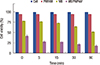
We examined the effect of PDT on MCF-7 cells in the presence of 0.6 µM methylene blue. The results showed that the cell viability decreased from 90% to 77% when the laser irradiation time increased from 0 to 2 hours, respectively (Figure 6). Similar experimental conditions have been used to investigate the influence of methylene blue loaded onto microgel PNIPAM on MCF-7 cells. As expected, loaded methylene blue-loaded microgel inhibited the growth of MCF-7 more effectively than did the methylene blue solution alone. The viability of MCF-7 cells decreased from 88% to 20% upon irradiation 0 to 2 hours, respectively. Hence, the efficiency of PDT proved higher in the presence of methylene blue-loaded microgel than that in the presence of methylene blue alone, even though the concentration of methylene blue was the same in both cases (0.6 µM).
In the control experiments, the viability of MCF-7 cells was not affected by PDT. A fairly high concentration (25 µM) of methylene blue was also tested for PDT, and considerable dark toxicity was observed. We found that cell viability decreased to 78% in the presence of 25 µM methylene blue even without laser irradiation. However, under similar conditions (i.e., no irradiation), cell viability markedly decreased (down to 42%) when methylene blue-loaded microgel was used (Figure 7). Cell viability reached 28% after irradiation for 5 minutes when 25 µM methylene blue was loaded onto the PNIPAM microgel, while the cell viability was reduced to 20% only after 2 hours when 0.6 µM methylene blue was loaded onto the PNIPAM microgel. For PDT, the ideal photosensitizer should show minimum dark toxicity. We found that 25 µM methylene blue-loaded microgels had 58% dark toxicity, while 0.6 µM methylene blue-loaded microgels showed only 12% dark toxicity.
We have synthesized PNIPAM microgel and its functionalized PNIPAm-co-PAA form for the encapsulation of methylene blue as a drug to be used in breast cancer treatment by PDT. The PNIPAM polymer is temperature sensitive and its LCST is 32℃ in phosphate buffer and 34℃ in deionized water. Previous studies have reported that the addition of salts decreases the LCST due to the salting out effect [22]. The effect of pH on the PNIPAM particle size was also investigated. At high pH, the majority of the AAc groups in the PNIPAM-co-PAA microgel were deprotonated because the pKa of polyacrylic acid is approximately 4.25. This property increases the size of the PNIPAM-co-PAA microgel at high pH due to charge repulsion among the AAc groups and added osmotic pressure (Donna effect) arising from the incorporated counter ions [23,24].
We found that the anionic PNIPAM-co-PAA microgel encapsulated a low amount of cationic drug compared to the nonfunctionalized PNIPAM microgel. This finding indicated that electrostatic interactions play an important role in loading the drug. The PNIPAM-co-PAA microgel uptakes lower amount of drug than does the nonfunctionalized PNIPAM microgel because the cationic drug binds only to the AAc groups localized at or near the surface of the microgel surface [22]. When the cationic drug binds to the anionic AAc groups in the PNIPAM-co-PAA microgel, a collapsed "skin layer" is formed at the surface of the PNIPAM-co-PAA microgel, which inhibits the diffusion of additional drug into the bulk of the PNIPAM-co-PAA microgel. Furthermore, it has also been reported that increasing the functional groups of the PNIPAM-co-PAA microgel further decreases the uptake of drug. Hence, acid-base interactions do not play a critical role in the uptake capacity of anionic microgels for cationic drugs. This results are reasonable because, in the functionalized PNIPAM-co-PAA microgel, methylene blue or other drugs are mostly adsorbed on the skin layer or on the surface of the microgel, whereas in the nonfunctionalized PNIPAM microgel drugs are present mostly on the core of the microgel [17]. We speculated that the PNIPAM microgel protected the methylene blue effectively and prevented it from degradation.
Major problems in PDT are represented by the fact that nanoparticles cannot encapsulate high doses of anticancer drugs and cannot retain drugs for a long time. Calculations from the methylene blue loading experiment showed that the PNIPAM microgel encapsulated higher doses of methylene blue than did the PNIPAM-co-PAA microgel. In addition, the PNIPAM microgel retained the encapsulated methylene blue for long periods (Figure 7), which demonstrated the usefulness of this material in PDT. During PDT, methylene blue generates ROS, mainly singlet oxygen (1O2), because of the irradiation by laser at the wavelength of 663 nm. The concentration of ROS depends upon the irradiation time. ROS oxidize the cell's lipids, amino acids, and proteins, and induces necrosis and/or apoptosis of the cells [25]. However, methylene blue showed considerable dark toxicity on MCF-7 cells at high concentration, even without PDT. This dark toxicity of methylene blue is concentration dependent. Normally, methylene blue at concentrations greater than 0.6 µM shows significant dark toxicity toward MCF-7 cells when the cells are incubated for a long time [26]. These results indicated that we could employ low-dose methylene-loaded microgel for the successful PDT treatment of MCF-7 cells. Although the mechanism underlying the dark toxicity of methylene blue has not been clearly established, it was linked to the inhibition of soluble guanylate cyclase and nicotinamide adenine dinucleotide oxidation.
Polyacrylamide nanoparticles retains the photo-activity of methylene blue more effectively than only methylene blue alone, because the nanoparticles prevent the encapsulated methylene blue from being reduced by diaphorase enzymes, thereby retaining the photoactive form of methylene blue for efficient PDT treatment [27]. We also speculated that the PNIPAM microgel loaded with methylene blue more effectively accumulated in the nucleus of MCF-7 cells than did methylene blue alone, whereas, as previously reported, the free methylene blue molecules were accumulated in the mitochondria of the cell [28]. Accumulation of methylene blue within mitochondria decreased the PDT efficiency because of its conversion into its photochemically inactive leucomethylene blue form, thus leading to a low production of 1O2 within the MCF-7 cells.
In summary, we have employed non-functionalized PNIPAM and functionalized PNIPAM-co-PAA microgel to encapsulate methylene blue. Electrostatic interactions did not play an important role for encapsulation of methylene blue in the microgels. The anionic PNIPAM-co-PAA microgels encapsulated less amount of cationic drug compared to non-functionalized PNIPAM microgels because a collapsed "skin layer" formed at their surface, thus inhibiting the diffusion of additional methylene blue into the bulk of the microgel. Non-functionalized PNIPAM microgels released methylene blue more slowly than did functionalized PNIPAM-co-PAA, and effectively inhibited the growth of MCF-7 cells. We believe that the findings of this study add a valuable contribution to the investigation of breast cancer treatment based on photodynamic therapy.
Figures and Tables
 | Figure 1Dependence of hydrodynamic radius of nonfunctionalized poly(N-isopropylacrylamide) on temperature in 10 mM phosphate buffer (▓) and deionized (DI) water (▒) at pH 7.4. |
 | Figure 2Dependence of hydrodynamic radius of poly(N-isopropylacrylamide) and polyacrylic acid microgel in phosphate buffer pH 7.4 (▓) and pH 3 (▒). |
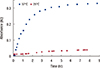 | Figure 3Release of methylene blue from functionalized of poly(N-isopropylacrylamide) and polyacrylic acid microgel at 37℃ (▓) and 25℃ (▒) in phosphate buffer, pH 7.4. |
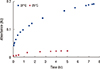 | Figure 4Release of methylene blue from non-functionalized of poly(N-isopropylacrylamide) and polyacrylic acid microgel at 37℃ (▓) and 25℃ (▒) in phosphate buffer, pH 7.4. |
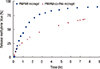 | Figure 5Comparison of methylene blue release from nonfunctionalized poly(N-isopropylacrylamide) (PNIPAM) (▓) and functionalized PNIPAM and polyacrylic acid (PNIPAM-co-PAA) (▴) microgel at 37℃. |
Notes
References
1. Ahmad A. Breast Cancer Metastasis and Drug Resistance: Progress and Prospects. New York: Springer;2013. p. 19–20.
2. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602.

3. Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007; 1776:86–107.

4. Siegel MM, Tabei K, Tsao R, Pastel MJ, Pandey RK, Berkenkamp S, et al. Comparative mass spectrometric analyses of Photofrin oligomers by fast atom bombardment mass spectrometry, UV and IR matrix-assisted laser desorption/ionization mass spectrometry, electrospray ionization mass spectrometry and laser desorption/jet-cooling photoionization mass spectrometry. J Mass Spectrom. 1999; 34:661–669.

5. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3:380–387.

6. Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008; 53:R61–R109.

7. Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008; 26:612–621.

9. Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999; 4:507–517.

10. Tuite EM, Kelly JM. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. J Photochem Photobiol B. 1993; 21:103–124.
11. Orth K, Beck G, Genze F, Rück A. Methylene blue mediated photodynamic therapy in experimental colorectal tumors in mice. J Photochem Photobiol B. 2000; 57:186–192.

12. Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008; 7:771–782.

13. Tang W, Xu H, Park EJ, Philbert MA, Kopelman R. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem Biophys Res Commun. 2008; 369:579–583.

14. Derycke AS, de Witte PA. Liposomes for photodynamic therapy. Adv Drug Deliv Rev. 2004; 56:17–30.

15. Regehly M, Greish K, Rancan F, Maeda H, Böhm F, Röder B. Water-soluble polymer conjugates of ZnPP for photodynamic tumor therapy. Bioconjug Chem. 2007; 18:494–499.

16. Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, et al. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J Am Chem Soc. 2003; 125:7860–7865.

17. Barbucci R, Magnani A, Consumi M. Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules. 2000; 33:7475–7480.

18. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986; 46(12 Pt 1):6387–6392.
19. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001; 41:189–207.

20. Grabstain V, Bianco-Peled H. Mechanisms controlling the temperature-dependent binding of proteins to poly(N-isopropylacrylamide) microgels. Biotechnol Prog. 2003; 19:1728–1733.

21. Schmitz KS. An Introduction to Dynamic Light Scattering by Macromolecules. Oxford: Academic Press;1990. p. 11–42.
22. Hoare T, Pelton R. Impact of microgel morphology on functionalized microgel-drug interactions. Langmuir. 2008; 24:1005–1012.

23. Fernández-Nieves A, Fernández-Barbero A, Vincent B, de las Nieves FJ. Charge controlled swelling of microgel particles. Macromolecules. 2000; 33:2114–2118.

24. Deka SR, Quarta A, Di Corato R, Falqui A, Manna L, Cingolani R, et al. Acidic pH-responsive nanogels as smart cargo systems for the simultaneous loading and release of short oligonucleotides and magnetic nanoparticles. Langmuir. 2010; 26:10315–10324.

25. Komine C, Tsujimoto Y. A small amount of singlet oxygen generated via excited methylene blue by photodynamic therapy induces the sterilization of Enterococcus faecalis. J Endod. 2013; 39:411–414.

26. Khdair A, Gerard B, Handa H, Mao G, Shekhar MP, Panyam J. Surfactant-polymer nanoparticles enhance the effectiveness of anticancer photodynamic therapy. Mol Pharm. 2008; 5:795–807.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


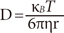
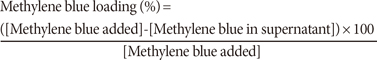
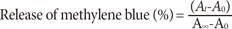
 XML Download
XML Download