Abstract
Purpose
We evaluated the efficacy of breast magnetic resonance imaging (MRI) for detecting additional malignancies in breast cancer patients newly diagnosed by breast ultrasonography and mammography.
Methods
We retrospectively reviewed the records of 1,038 breast cancer patients who underwent preoperative mammography, bilateral breast ultrasonography, and subsequent breast MRI between August 2007 and December 2010 at single institution in Korea. MRI-detected additional lesions were defined as those lesions detected by breast MRI that were previously undetected by mammography and ultrasonography and which would otherwise have not been identified.
Results
Among the 1,038 cases, 228 additional lesions (22.0%) and 30 additional malignancies (2.9%) were detected by breast MRI. Of these 228 lesions, 109 were suspected to be malignant (Breast Imaging-Reporting and Data System category 4 or 5) on breast MRI and second-look ultrasonography and 30 were pathologically confirmed to be malignant (13.2%). Of these 30 lesions, 21 were ipsilateral to the main lesion and nine were contralateral. Fourteen lesions were in situ carcinomas and 16 were invasive carcinomas. The positive predictive value of breast MRI was 27.5% (30/109). No clinicopathological factors were significantly associated with additional malignant foci.
Breast cancers can be multifocal and multicentric. In women with newly diagnosed breast cancer, 20% to 60% are believed to harbor additional ipsilateral malignant foci other than the index tumor, detected by clinical examination or conventional imaging [1,2,3]. Accurate determination of the tumor extent and presence of additional tumor foci can improve the surgical outcome in breast conservation surgery, by reducing the re-operation rate as well as the local recurrence rate. In contrast, only 1% to 3% of newly diagnosed women have synchronous contralateral breast cancer. These patients have a poor prognosis compared to those with unilateral breast cancer, indicating the importance of early detection of contralateral malignancies in newly diagnosed patients [4,5].
Breast magnetic resonance imaging (MRI) is increasingly used both in preoperative imaging studies in breast cancer patients and for screening in high-risk women because of its high sensitivity for breast cancer detection. Although mammography remains the standard method of diagnosis, considerable evidence has indicated that breast MRI is more sensitive than conventional imaging in identifying additional cancer foci that would otherwise have not been detected [6,7,8]. A recent meta-analysis showed that the addition of breast MRI increased the incremental detection rates (ICDRs) of additional malignancies by 16% in the ipsilateral breast. Additionally, MRI can detect contralateral breast cancer in up to 4.1% of primary breast cancer patients. Considering the substantial rate of ipsilateral tumor recurrence after breast conservation surgery and the prognostic importance of contralateral breast cancer, it is hoped that the use of MRI can improve the treatment outcome of breast cancer patients [5,7,9].
In women with mammographically dense breasts, conventional imaging for breast cancer often includes breast ultrasonography. Furthermore, preoperative ultrasonography-guided procedures such as needle biopsies for suspicious lesions are commonly used. The benefit of breast MRI in terms of improved ICDRs in breast cancer patients who have undergone ultrasonography and mammography is currently unclear. Clinical studies involving a small number of patients have shown that MRI is more sensitive than ultrasonography [10,11,12]. However, no study has addressed this issue in a large number of patients.
We retrospectively reviewed the records of 3,936 breast cancer patients who underwent surgery at the Seoul National University Hospital between August 2007 and December 2010. During this period, patients with newly diagnosed breast cancer underwent breast MRI if there were no contraindications such as renal insufficiency. Patients with unilateral breast MRI and those who received neoadjuvant chemotherapy or underwent excisional biopsy for cancer diagnosis were excluded. As our aim was to examine the true additional value of breast MRI compared to mammography and ultrasonography, patients were included only when the initial diagnostic mammography and ultrasonography results prior to breast MRI were available. Patients were excluded if they underwent breast ultrasonography before referral to our institution, because of the lack of initial imaging findings. A total of 1,038 patients were examined. The study protocol and the use of information from our institutional database were approved by the Institutional Review Board of the Seoul National University Hospital (IRB number, 1106-070-366).
All breast MRIs were performed using a 1.5-T system (Signa®; General Electric Medical Systems, Milwaukee, USA), as described previously [15]. Fat-suppressed T2-weighted fast spin-echo images (repetition time [TR]/echo delay time [TE], ranging from 5,500 to 7,150/82 ms; matrix, 256×160; field of view, 200×200 mm2; slice thickness, 1.5 mm), and dynamic contrast-enhanced images, including one precontrast and five postcontrast sagittal images using a fat-suppressed T1-weighted 3D fast spoiled gradient echo sequence (TR/TE, 6.5/2.5 ms; matrix, 256×160; flip angle, 10°; field of view, 200×200 mm; slice thickness, 1.5 mm) were obtained. Second-look bilateral ultrasonography was performed in all patients prior to surgery. Bilateral whole-breast ultrasonography was performed with 10 or 12 MHz linear transducers for high image resolution by an experienced radiologist (Voluson 730, Kretz-Medison, Zipf, Austria; HDI 5000, Advanced Technology Laboratories, Bothell, USA). This procedure is routinely performed not only in cancer patients, but also for population screening, as Korean women have small and relatively dense breasts, which makes it a feasible diagnostic tool.
Lesions undetected by conventional methods (mammography and ultrasonography) but detected by sequentially performed contrast-enhanced breast MRI were defined as additional lesions. Lesions within 0.5 cm of the index tumor, which would not have affected the surgical extent were considered daughter nodules and were not counted. Lesions were assessed according to the Breast Imaging-Reporting and Data System (BI-RADS) classification (Category0 [C0], incomplete study; C1, negative; C2, benign; C3, probably benign; C4, suspicious malignancy; C5, highly suggestive of malignancy; C6, proven malignancy). Additional malignancy was defined as pathologically confirmed in situ or invasive carcinoma. The incidence of additional malignancy, positive predictive value (PPV), and false positive (FP) rate were analyzed. PPV was defined as the number of true positive (TP) cases/the number of TP+FP cases. Lesions were classified according to the BI-RADS, and class 4 or 5 lesions were considered suspicious of malignancy. All suspicious lesions were re-evaluated by second-look mammography and ultrasonography before surgery. Most patients opted for lesion examination by excisional biopsy in a one-stage surgery because of the possible time delay between diagnosis and surgery to confirm the pathology of the additional suspicious lesion. As most of the lesions were identified by focused second-look ultrasonography, ultrasound-guided wire localization or skin marking was performed if they were not included in the initially planned surgery for the primary tumor. Suspicious additional lesions were surgically excised at the time of the curative operation, either by separate excisional biopsy or by extended surgery. All surgical specimens were examined by serial sectioning, and the pathologic positivity of the surgically removed MRI-detected lesions was assessed by carefully matching the pathology reports and MRI findings. The association between clinicopathological factors and the risk of additional malignancy was evaluated.
Differences in the distribution of categorical variables were analyzed using the Pearson chi-square test, whereas continuous variables were compared using the Student t-test. A stepwise logistic regression model was used for multivariate analysis. All statistical analyses were performed using the SPSS version 19.0 software package (SPSS Inc., Chicago, USA), with p<0.05 considered statistically significant.
The demographic and clinicopathological characteristics of the study population are shown in Table 1. The mean age of the patients was 49.57±10.17 years and 569 of the 1,038 women (54.8%) were premenopausal. The mean BMI was 23.54±3.15 kg/m2. On mammography, 714 women (68.8%) had a breast density of grade 3 or 4. The mean tumor size was 1.95±1.69 cm with 44 women (4.3%) having a lobular carcinoma component.
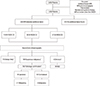
Overall, breast MRI detected additional lesions in 228 of the 1,038 patients (22.0%) (Table 2, Figure 1). Of these 228 lesions, 109 (47.8%) were suspicious of malignancy (BI-RADS class 4 or 5) on breast MRI and second-look ultrasonography. Fourteen MRI-defined C3 lesions and 12 MRI-defined C0 lesions were upstaged to BI-RADS C4 after second-look ultrasonography, resulting in 109 additional suspicious lesions (Figure 1). Four lesions were unidentifiable by second-look ultrasonography, and these lesions were closely observed without pathologic examination. There was no sign of local recurrence after more than 48 months of follow-up in these four cases. Of the 109 suspicious lesions, 51.4% (56/109) were in the ipsilateral breast and 48.6% (53/109) were in the contralateral breast. Pathology results revealed the presence of malignancy in 30 MRI-detected lesions (2.9%) (21 in the ipsilateral breast and nine in the contralateral breast). Of these tumors, 14 were in situ carcinomas and 16 were invasive carcinomas. The PPV of breast MRI was 27.5% (30/109).
The combination of breast MRI and second-look ultrasonography resulted in ICDRs of 2.0% (21/1,038) in the ipsilateral breast and 0.9% (9/1,038) in the contralateral breast, resulting in an overall ICDR of 2.9%. The overall PPV was 27.5% (30/109); the PPVs for ipsilateral and contralateral breast tumors were 37.5% (21/56) and 17.0% (9/53), respectively. The overall TP:FP ratio was 0.38 (30:79):0.60 (21:35) for the ipsilateral breast and 0.20 (9:44) for the contralateral breast.
We also assessed the demographic and clinical factors associated with the increased risk of MRI-detected additional malignancies (Table 3). In univariate analysis, age, mammographic breast density, index tumor size, histological grade, estrogen receptor status, and lymph node metastasis were not significantly associated with the detection of additional malignant lesions. Logistic regression analysis, including the patient's age, lobular component, and mammographic breast density, did not identify any significantly associated factor either; age ≤50 years was the only factor that tended to be associated with MRI-detected additional malignancy (odds ratio, 2.16; 95% confidence interval, 0.89-5.29; p=0.090) (Table 4). These factors were selected for logistic regression analysis with the assumption that young patients with a high breast density could possibly benefit from breast MRI and because lobular carcinoma is more likely to involve multiple tumors. As age was significantly correlated with density (Pearson correlation coefficient=-0.409), forward selection analysis was performed, and all three factors had adjusted p-values of >0.05 (Table 4).
The breast conservation rate was 53.3% (16/30) for TP cases and 58.2% (31/79) for FP cases, compared to 59.8% (484/810) for patients without additional lesions (p=0.647).
Advances in MRI technology have enabled improved detection
and spatial definition of breast cancer, which has led to the wide use of breast MRI in breast cancer diagnosis and management [16]. This increase in the use of breast MRI is based on the assumption that the high sensitivity could result in more accurate assessments of tumor extent and burden, including the presence of additional malignant foci. This, in turn, could result in favorable clinical outcomes in terms of re-excision and in-breast tumor recurrence rates, among other outcomes [6,14,16,17,18].
We evaluated the ICDR associated with preoperative breast MRI for the detection of additional malignant foci in the affected breast and in the contralateral unaffected breast in a large number of Asian breast cancer patients who also underwent breast ultrasonography and mammography. Although it was a large study, there were limitations to this study. Whether MRI resulted in more extensive surgery is unclear, because information on the initial surgical plan before performing breast MRI was generally unavailable. Additionally, the survival difference could be efficiently assessed, as the number of cases with additional malignant foci was small. As MRI-guided biopsy procedures were not performed during the study period, the whole study population underwent surgical excision of the suspicious lesions for pathologic confirmation. The PPV and TP:FP of breast MRI in terms of detecting additional malignancies should be re-evaluated in a setting with MRI-guided biopsies.
Our results show that MRI had limited efficacy in detecting additional malignancies with an ICDR of 2.0% for the ipsilateral breast and 0.9% for the contralateral breast. These results are in contrast with those of a meta-analysis of 19 studies, which showed that the overall ICDR in the affected breast of 2,610 patients was 16% [7]. Another review of 22 studies, involving 3,253 patients, reported that the ICDR for synchronous contralateral breast cancer was 4.1% (131/3,253) [4]. These discrepancies may have been due to the use of bilateral whole breast ultrasonography in our entire study population. We have previously reported that the additional use of preoperative ultrasonography detected a significant number of additional malignancies, when compared to clinical examination and mammography (14% for the ipsilateral breast and 4% for the contralateral breast) [19]. When these values were added to the ICDRs resulting from breast MRI in our study, the sum of ICDRs by ultrasonography and MRI were almost equal to the ICDRs of MRI without ultrasonography in the meta-analyses (2.0%+14% vs. 16% for the ipsilateral breast and 0.9%+4% vs. 4.1% for the contralateral breast). These results suggest that breast MRI after bilateral whole breast ultrasonography, even when performed by an experienced radiologist, has little incremental benefit in detecting additional malignant foci. Moreover, with continuous advances in ultrasonography technology, the additional value of a combination of ultrasonography and mammography might be even higher compared to mammography alone, because mammography alone offers little information given the high breast density of the Korean population.
In the logistic regression model to identify factors associated with the benefits of preoperative breast MRI, we found that patients aged ≤50 years showed a tendency of having additional malignancies detected by MRI. However, this tendency was negated after adjustments for confounding factors (p=0.196) and the ICDR was still low in this group (>50 years, 3.5% vs. 2.2%; p=0.090). Therefore, it remains unclear whether routine breast MRI after mammography and breast ultrasonography can be justified in this age group. Furthermore, the survival benefit associated with extirpating MRI-detected tumors is not clear based on our knowledge that many of the occult malignant foci in the remnant breast are not clinically relevant [20,21,22,23,24]. To date, no randomized trial has assessed the impact of presurgical breast MRI on long-term patient outcomes. Fischer et al. [25] have shown that MRI staging was not associated with any difference in the 8-year rates of local and systemic recurrence. A recent study including 756 patients also demonstrated the lack of a survival difference when preoperative MRI was used [26]. Another concern is the rate of FP cases, leading to unnecessary procedures and delaying definitive treatment [27]. Approximately 5.5% of women undergo extensive surgery (wider excision or mastectomy) because of FP lesions [7]. Moreover, preoperative breast MRI has been found to increase the rate of mastectomy [27]. In our study, we found that the overall TP:FP ratio was 0.38 (30:79; 0.60 for the ipsilateral breast and 0.20 for the contralateral breast). Although we did not observe a significant increase in the mastectomy rate, a high incidence of FP cases raises concerns of potentially increased incidences of wider excisions and poor cosmetic outcomes. MRI-guided biopsy should be the initial tool for pathologic confirmation even though the FP and false negative rates may be higher than for surgical excisional biopsies owing to the nature of targeted-needle biopsies.
Preoperative breast MRI after breast ultrasonography resulted in a minimal increase in the ICDR of additional malignant foci in the ipsilateral or contralateral breast. Clinicopathological factors were not associated with the presence of additional malignant foci. This suggests that the benefits of performing preoperative breast MRI are minimal. Routine use of preoperative breast MRI should be discouraged when good quality bilateral whole-breast ultrasonography is performed by an experienced radiologist.
Figures and Tables
Figure 1
Additional lesions detected by preoperative breast MRI Among the 1,038 patients included in the analysis, MRI detected additional lesions in 228 patients. A total of 109 were suspicious lesions (BI-RADS category 4 or 5) after second-look targeted ultrasound and 30 were confirmed malignancy.
MRI=magnetic resonance imaging; SNUH=Seoul National University Hospital; BI-RADS=Breast Imaging-Reporting and Data System; NED=No evidence of disease.
*Benign likely: BI-RADS category 3 lesion both in breast MRI and second-look ultrasonography; †Suspicious malignancy: BI-RADS category 4 or higher lesion after second-look ultrasonography including upstaged; ‡Unknown: lesions unidentifiable after second-look ultrasonography; §Pathologic confirmation: all suspicious lesions were surgically excised and pathologic confirmation was done.
Notes
References
1. Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer. 1996; 74:820–824.

2. Lagios MD. Multicentricity of breast carcinoma demonstrated by routine correlated serial subgross and radiographic examination. Cancer. 1977; 40:1726–1734.

3. Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer. 1985; 56:979–990.

4. Brennan ME, Houssami N, Lord S, Macaskill P, Irwig L, Dixon JM, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009; 27:5640–5649.

5. Carmichael AR, Bendall S, Lockerbie L, Prescott R, Bates T. The long-term outcome of synchronous bilateral breast cancer is worse than metachronous or unilateral tumours. Eur J Surg Oncol. 2002; 28:388–391.

6. Liberman L. Breast MR imaging in assessing extent of disease. Magn Reson Imaging Clin N Am. 2006; 14:339–349. vi

7. Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008; 26:3248–3258.

8. Smith RA. The evolving role of MRI in the detection and evaluation of breast cancer. N Engl J Med. 2007; 356:1362–1364.

9. Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011; 21:26–34.

10. Francis A, England DW, Rowlands DC, Wadley M, Walker C, Bradley SA. The diagnosis of invasive lobular breast carcinoma: does MRI have a role? Breast. 2001; 10:38–40.

11. Schelfout K, Van Goethem M, Kersschot E, Verslegers I, Biltjes I, Leyman P, et al. Preoperative breast MRI in patients with invasive lobular breast cancer. Eur Radiol. 2004; 14:1209–1216.

12. Davis PL, Staiger MJ, Harris KB, Ganott MA, Klementaviciene J, McCarty KS Jr, et al. Breast cancer measurements with magnetic resonance imaging, ultrasonography, and mammography. Breast Cancer Res Treat. 1996; 37:1–9.

13. Hillman BJ. Do we need randomized controlled clinical trials to evaluate the clinical impact of breast MR imaging? Magn Reson Imaging Clin N Am. 2006; 14:403–409. vii–viii.

14. Morrow M, Freedman G. A clinical oncology perspective on the use of breast MR. Magn Reson Imaging Clin N Am. 2006; 14:363–378. vi

15. Kim JY, Cho N, Koo HR, Yi A, Kim WH, Lee SH, et al. Unilateral breast cancer: screening of contralateral breast by using preoperative MR imaging reduces incidence of metachronous cancer. Radiology. 2013; 267:57–66.

16. Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009; 59:290–302.

17. Schnall M. MR imaging evaluation of cancer extent: is there clinical relevance? Magn Reson Imaging Clin N Am. 2006; 14:379–381. vii

18. Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007; 16:Suppl 2. S34–S44.

19. Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology. 2002; 224:569–576.

20. Morris AD, Morris RD, Wilson JF, White J, Steinberg S, Okunieff P, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. Cancer J Sci Am. 1997; 3:6–12.
21. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.

22. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.

23. Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003; 98:697–702.

24. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000; 92:1143–1150.

25. Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999; 213:881–888.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download