Abstract
Purpose
The objective of this study was to investigate the influence of radiotherapy on the cosmetic outcome after immediate breast reconstruction using an absorbable mesh in breast cancer.
Methods
From July 2008 to July 2009, 35 breast cancer patients who received immediate breast reconstruction with absorbable mesh insertion at the time of breast conserving surgery followed by radiotherapy were retrospectively studied.
Results
In 91% of cases there was an excellent or good cosmetic outcome before the initiation of radiotherapy, and in 8.6% the outcome was fair at this point. However, 6 months after surgery and irradiation, the rate of excellent to good cosmetic outcomes had decreased to 60% and fair outcomes had increased to 25.7%. Contrary to the decreased rate of good cosmetic outcomes from 65.7% to 42.9% at 1 year after operation, the rate of fair to poor outcomes considerably increased from 8.6% to 57.1%. The significant factors affecting cosmetic outcomes were pathology, specimen volume, and the estimated percentage of breast volume excised (EPBVE). Chemotherapy affected the cosmetic outcome at borderline significance level. Age, breast volume tumor site, insertion of drain, radiation dose, and time elapsed between surgery and radiotherapy were not significantly associated with the cosmetic outcome.
Since breast-conserving surgery (BCS) followed by whole breast irradiation has become accepted as the standard treatment [1,2], the rate of conserving surgery in Korea has gradually increased to 50% of breast cancer surgery [3]. Although BCS achieves good cosmetic outcomes compared with mastectomy, the wide excision necessary for the negative resection margin is associated with poor cosmetic results, depending on the tumor size, breast volume, and location [4,5]. In order to optimize the balance between a safe resection margin and cosmetic outcomes in BCS, oncoplastic techniques have been introduced in recent years [6-8]. Oncoplastic techniques using autologous tissues are generally believed to allow for superior cosmetic results, but are substantially more complex and time consuming than procedures using prosthetic material [9,10].
In 2003, an absorbable synthetic polyglycolic acid mesh was first proposed as a filling material for the volume defect in BCS [11,12]. In 2009, a Korean national survey revealed that 74.1% of surgeons had used an absorbable mesh due to the simplicity and time saving aspects of the technique [13]. Although absorbable mesh insertion has been reported to be a technically feasible and time sparing procedure [14], a significant rate of infection, chronic pain and subsequent removal of the mesh or surgical intervention have recently been reported as follow-up time progresses [15,16].
Most published studies identify radiotherapy as adversely affecting the cosmetic outcome, particularly in implant-based reconstructions, by precipitating the inflammatory reaction of the implant to the surrounding tissues [17-19]. However few studies have reported on the influence of radiotherapy on the cosmetic results after absorbable mesh insertion into the volume defect space of the operated breast. The objective of the current study was to investigate the influence of radiotherapy on the cosmetic outcome after immediate breast reconstruction using an absorbable mesh in breast cancer.
From July 2008 to July 2009, 35 breast cancer patients who received immediate breast reconstruction with an absorbable mesh insertion at the time of BCS followed by chemotherapy and radiotherapy at our institution were retrospectively studied. The study was approved by the IRB No. 13-006, and patients gave their written consent for their participation. Photograph was taken in patients who agreed to be recorded for the presentation. Patient characteristics and follow-up results including cosmetic outcomes, clinical and radiological findings are reviewed and shown in Table 1.
The absorbable mesh (polyglactin vicryl mesh®; Ethicon, Johnson and Johnson, Somerville, USA) was folded like a fan and wrapped using the absorbable adhesion barrier Interceed® (Ethicon, Johnson and Johnson), which comprises oxidized regenerated cellulose. The mesh and intercede complex was inserted into the volume defect space after surgical removal of the lesion for breast conservation [11]. A drain was inserted into the volume defect space in eight patients.
All patients received radiotherapy to the whole breast using tangential fields with subsequent electron boost to the tumor bed at a total dose ranging from 50.4 to 66.6 Gy. Mammography and ultrasound were used for the postoperative radiologic survey. Chemotherapy (anthracycline and taxane) was administered prior to operation in six patients and 15 patients received chemotherapy after the operation.
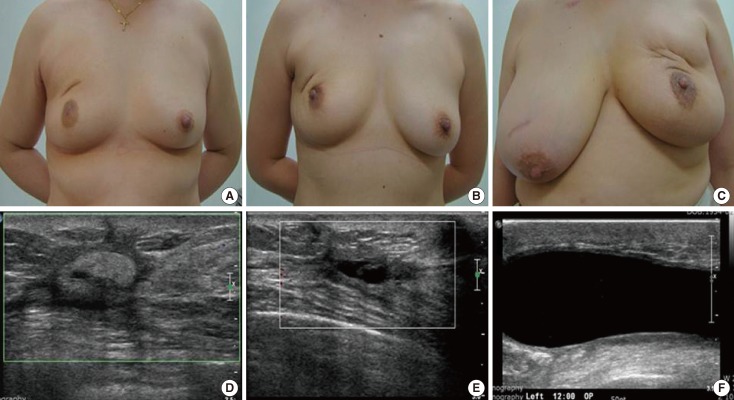
The cosmetic outcome was evaluated and scored by two physicians, a surgeon who carried out the operation and a radiation oncologist, based on the Harvard Breast Cosmesis Scale: excellent (almost identical to untreated breast), good (minimal difference between breasts, satisfactory symmetry and distortion), fair (obvious difference, acceptable asymmetry and distortion), and poor (major difference, noticeable asymmetry and distortion) (Figure 1) [20]. The appearance of the breast using a photograph taken after surgery was rated during follow-up visits. The cosmetic outcome assessment was repeated at three different time point after operation: before the initiation of irradiation, at 6 months and 1 year postoperation (Table 2).
The following factors were analyzed to evaluate their association with the cosmetic outcome: age, pathology, specimen volume, breast size, estimated percentage of breast volume excised (EPBVE), tumor site, radiation dose, insertion of drain, chemotherapy and the time elapsed between surgery and radiation (Table 3). EPBVE represents the ratio of excised breast volume to whole breast. Each whole breast volume was calculated based on presurgical mammography using the formula: π1/3HR2, where H is breast height and R is breast radius. The volume of excised breast was measured with a ruler. Fisher's exact test for discrete variables and ANOVA for continuous variables were used to determine the statistical significance of the data. SPSS version 18.0 (IBM, Armonk, USA) was used for all analyses.
The patient characteristics are summarized in Table 1. The median follow-up period was 23 months. The median age was 47 years old, with a median tumor size of 1.4 cm. Invasive ductal carcinoma was found in 22 patients (62.8%) and the upper outer quadrant was the most tumor common site (45.7%). The median breast volume was 704.4 cm3. The excised specimen tissue showed a median volume of 209 cm3. The ratio of the specimen volume to the breast volume (EPBVE) was 21.9% as a median value. The median time elapsed between surgery and radiotherapy was 5 weeks.
The rate of good to excellent cosmetic outcomes was 91.4% and that for fair outcome was 8.6% before the initiation of radiotherapy. However, 6 months after surgery and irradiation, the rate of excellent to good cosmetic outcomes had decreased to 60% and that for fair had increased to 25.7%. Contrary to the decreased rate of good cosmetic outcomes from 65.7% to 42.9% at 1 year postoperation, the rate of fair to poor was considerably increased from 8.6% to 57.1% (Table 2).
The significant factors affecting the cosmetic outcome were pathology (p=0.027), specimen volume (p=0.005), and the EPBVE (p=0.008). Chemotherapy affected the cosmetic outcome at a level of borderline significance (p=0.064 in neoadjuvant chemotherapy group and p=0.051 in postoperative adjuvant chemotherapy group). Age, breast volume, tumor site, insertion of drain, radiation dose, and the time elapsed between surgery and radiotherapy were not significantly associated with the cosmetic outcome (Table 3).
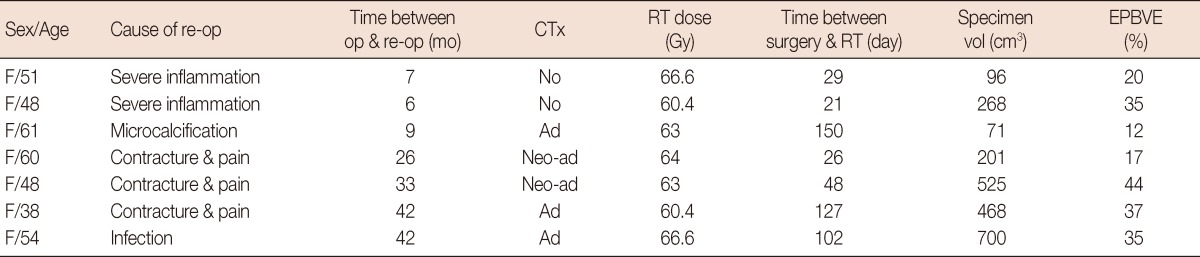
Among the seven patients (20%) who had to be reoperated on due to mesh complication, partial mastectomy for mesh removal was performed in two patients due to severe inflammation and seroma at around 6 months postoperation (Table 4). One patient who showed microcalcification at the mesh inserted site on mammography at 9 months after BCS received excision of the lesion for evaluation. The pathologic findings of the removed lesion revealed foreign body reaction and fat necrosis with microcalcification. Two patients received partial mastectomy with lattisimus dorsi muscle flap 2 years postoperatively and one patient underwent the same operation 3 years postoperatively due to severe contracture and pain. The mesh and surrounding tissue had to be excised in one patient who showed infection at the implanted site 3 years postoperation.
Ten patients had persistent pain and eight complained of itching sensations at the implanted site at 1 year follow-up. Ultrasonography revealed various amount of seroma and some extent of parenchymal distortion at the mesh inserted site in these patients.
The simplicity and time sparing benefits of absorbable mesh insertion into the volume defect caused by lumpectomy are reasons for the utilization of a mesh in breast reconstruction surgery. According to a Korean national survey, 74.1% of surgeons responded that absorbable mesh is used during breast reconstruction surgery [13]. Previous research on biomechanical materials using an absorbable mesh demonstrated a pronounced level of inflammation and an increased level of connective tissue formation at the interface [21]. In fact, the inflammatory reaction and increased level of tissue formation induced by an absorbable mesh, that is a polyglactin vicryl prosthesis, in the volume defect space is a necessary part of the process of cicatrization of surrounding breast tissues, supporting structures to prohibit deformity or postoperative dimpling [22]. The inflammatory reaction, may however increase the probability of infection in the implanted site, which is closely related with breast pain, contracture, skin edema, and the cosmetic outcome. Furthermore, irradiation to the mesh implanted breast synergistically precipitates the inflammatory reaction, which increases the probability of infection and fibrotic change [17-19]. As a consequence, the implanted site may be replaced as fibrotic granuloma, even when the mesh is absorbed. Therefore, though the cosmetic outcome may be scored as good, a lump may be palpable in the mesh implanted breast (Figure 1A).
According to our study, chemotherapy also may participate in the induction of the inflammatory reaction around the implanted site, although the effect of chemotherapy on inflammation was only borderline significance in both the neoadjuvant and adjuvant sequence.
Polyglactin vicryl is reported to lose half of its tensile strength within 2 to 3 weeks, and is fully absorbed after 3 months, allowing the operated breast to preserve an ideal shape [23]. According to early studies on the outcomes of using absorbable mesh for immediate breast reconstruction with a short-term follow-up period in Korea, most authors have reported satisfactory cosmetic outcomes with no major complications [14,22,24]. Other authors showed that the use of mesh in breast surgery can enhance the cosmetic outcomes without inducing visible deformities or infection after a mean follow-up period of 30 months [25,26]. They restricted the reconstruction with mesh only to the benign breast condition. The excellent cosmetic outcome in their study thus accounted for no additional irradiation to the breast.
However, Cho et al. [15] reported that infection generally develops 3 months after surgery and the probability of infection in patients implanted with absorbable mesh was reported to be 10.3%, which is a relatively higher rate than previous reported rate of 3% to 7% [13]. Koo et al. [16] found that severe pain or discomfort, edema, and recurrent fluid collection occurred in 26.5% of cases. The median follow-up period of this study was 18 months, which provided a longer window for observation of the later side effect induced by mesh and radiotherapy. Earlier studies with shorter follow-up period on the safety of mesh insertion appear to have underestimated the risk of mesh insertion, particularly in the patients who are irradiated [14,22,24]. The late side effect of radiotherapy may be progressively manifested years later [27]. We also found that the cosmetic outcome deteriorated after 1 year postsurgery (Table 2).
In this study, the cosmetic outcome was the end point to determine whether absorbable mesh insertion to the breast is suitable for oncoplastic reconstruction in patients scheduled to be irradiated. The 25.7% excellent and 65.7% good cosmetic outcomes at 3 months postoperation decreased to 0% excellent and 42.9% good cosmetic outcomes post 1-year follow-up (Table 2). However, recent data on the cosmetic outcomes of conventional BCS and adjuvant radiation without oncoplastic intervention showed 71% good to excellent outcomes at 5 years (27% excellent and 44% good) [28]. The reduction of excellent or good cosmetic outcomes from 91.4% to 42.9% at 1 year follow-up implies that adjuvant radiation contributed to the rapid deterioration of the cosmetic outcome. The low rate of good to excellent cosmetic outcomes (42.9%) and the rapid deterioration of the cosmetic outcome at 1 year, and the high rate of reoperation (20%) after conventional BCS and adjuvant radiation suggest that absorbable mesh insertion is not recommendable as an oncoplastic technique.
These results demonstrate that the dynamic processes of complex tissue remodeling and healing, both before and after radiotherapy within the lumpectomy cavity implanted with an absorbable mesh and the surrounding tissue of the breast, occur continuously over a 1 year follow-up period. Although deterioration of the cosmetic outcome as time progresses would be expected to a certain extent, as fibrosis is well known to be a late normal tissue effect resulting from radiotherapy in nonimplanted breast, the rapid deterioration of the cosmetic outcome over 1 year reveals that absorbable mesh synergistically increases the inflammatory process and the probability of fibrosis with radiotherapy. Whitfield et al. [27] also reported that capsular contracture steadily increased even until 4 years in patients who received radiotherapy after immediate breast reconstruction with an implant or nonimplant autologous flaps.
Koo et al. [16] reported that mesh removal was inevitable in 3 of 34 patients (8.8%) for recurrent mastitis. The authors indicated noticeable side effects and argued that the oncologic safety of the procedure is unconfirmed and concluded that mesh insertion should be considered cautiously only in selected cases. The proportion of patients receiving irradiation in Cho et al. [15] and Koo et al. [16] was over 94%. The higher rate of complication in contrast to earlier reports of breast reconstruction with absorbable mesh can be attributed to the increased inflammation induced by irradiation at the site of the implanted breast. Most published studies identify radiotherapy as adversely affecting the cosmetic outcome, particularly in implant-based reconstructions [18].
Regarding the timing of radiotherapy in mesh implanted patients, although Cho et al. [15] reported that the initiation of radiotherapy after 100 days of surgery decreased the infection rate significantly, we could not find a significant relationship between the time elapsed between surgery and radiotherapy and the cosmetic outcome. In our data, 74.3% of patients started radiotherapy 3 months after surgery. However, the delay of radiotherapy for 3 months until the mesh is fully absorbed to avoid the synergistic effect of inflammation with radiation may contribute to better cosmetic outcomes [23]. Studies on the timing of radiotherapy that delayed, the start of breast irradiation up to 16 weeks from the definitive surgery showed that this does not appear to affect local recurrence in low risk breast cancer patient [29]. Therefore delay of radiotherapy initiation for about 3 months after surgery may be considered until the resolution of inflammation around the lumpectomy cavity implanted with mesh is fully reached, so as to minimize the synergistic inflammatory effect induced by radiation for the optimum cosmetic outcome.
The specimen volume and EPBVE were highly statistically significant, implying a higher rate of poor cosmetic outcomes in patients who had a larger volume of breast tissue removed (Table 3). In patients with a mean EPBVE ranging from 30% to 40%, the fair to poor cosmetic outcome was 57.1% at 1 year postoperation. The results of cosmetic outcome based on EPBVE provide a basis for determining the suitability of mesh implantation to correct the cavity defect after BCS. Our study shows that applying an absorbable mesh for the reconstruction of the breast in patients with a large breast volume excised, i.e., an EPBVE ranging from 30% to 40%, does not appear to provide a satisfactory cosmetic outcome. For a good cosmetic outcome in mesh implanted breast cancer patients who are scheduled to be irradiated, an appropriate EPBVE can be presumed to be in the range between 10% and 30%. However, a good cosmetic outcome may be achieved only with through the volume displacement technique of oncoplastic surgery without the insertion of any kind of prosthetic materials in patients with an EPBVE in the range between 10% and 30%.
The follow-up period of our study is too short to reach a definitive conclusion for the reason that the effects of radiation continue for years and cosmetic outcomes should be evaluated in the 5 to 10 year range. This implies that longer-term follow-up may be required to present guidelines designed minimize the unexpected adverse effects of mesh insertion, which is not confirmed as being safe for use in oncoplastic reconstruction. A limitation of our study was the relatively small size of patients, who came from one center.
In conclusion, applying an absorbable mesh for the immediate reconstruction of the breast is not suitable in patients with an EPBVE over 30% who are scheduled to be irradiated.
References
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241. PMID: 12393820.

2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232. PMID: 12393819.

3. Jung YS, Na KY, Kim KS, Ahn SH, Lee SJ, Park HK, et al. Nation-wide Korean breast cancer data from 2008 using the breast cancer registration program. J Breast Cancer. 2011; 14:229–236. PMID: 22031806.

4. Raja MA, Straker VF, Rainsbury RM. Extending the role of breast-conserving surgery by immediate volume replacement. Br J Surg. 1997; 84:101–105. PMID: 9043470.

5. Fujishiro S, Mitsumori M, Kokubo M, Nagata Y, Sasai K, Mise K, et al. Cosmetic results and complications after breast conserving therapy for early breast cancer. Breast Cancer. 2000; 7:57–63. PMID: 11029772.

6. Asgeirsson KS, Rasheed T, McCulley SJ, Macmillan RD. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005; 31:817–823. PMID: 16043322.

7. Quinn McGlothin TD. Breast surgery as a specialized practice. Am J Surg. 2005; 190:264–268. PMID: 16023443.

8. Brédart A, Petit JY. Partial mastectomy: a balance between oncology and aesthetics? Lancet Oncol. 2005; 6:130. PMID: 15737826.

9. Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000; 87:1455–1472. PMID: 11091232.

10. Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001; 19:1539–1569. PMID: 11230499.

11. Sanuki J, Fukuma E, Wadamori K, Higa K, Sakamoto N, Tsunoda Y. Volume replacement with polyglycolic acid mesh for correcting breast deformity after endoscopic conservative surgery. Clin Breast Cancer. 2005; 6:175. PMID: 16001998.

12. Sanuki J, Higa K, Wadamori K, Fukuma E. Filling method to defect part of mammary gland after breast-conserving therapy by using polyglycol mesh. J Jpn Surg Soc. 2003; 104:243.
13. Kim KS, Park MY, Kim WJ, Na KY, Jung YS, Choi YJ, et al. Nationwide survey of the use of absorbable mesh in breast surgery in Korea. J Breast Cancer. 2009; 12:210–214.

14. Lee JH, Hong YI, Jeong JH, Lee JI, Lee JH, Moon HJ, et al. Volume replacement with polyglactin 910 mesh for breast reconstruction after endoscopy-assisted breast conserving surgery for treating early breast cancer: the early results. J Breast Cancer. 2009; 12:193–198.

15. Cho JS, Shin SH, Park JY, Song YJ, Yi JM, Park MH, et al. Analysis of infections occurring in breast cancer patients after breast conserving surgery using mesh. J Breast Cancer. 2011; 14:328–332. PMID: 22323921.

16. Koo MY, Lee SK, Hur SM, Bae SY, Choi MY, Cho DH, et al. Results from over one year of follow-up for absorbable mesh insertion in partial mastectomy. Yonsei Med J. 2011; 52:803–808. PMID: 21786446.

17. Taylor CW, Horgan K, Dodwell D. Oncological aspects of breast reconstruction. Breast. 2005; 14:118–130. PMID: 15767181.

18. Benediktsson K, Perbeck L. Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg. 2006; 59:27–34. PMID: 16482787.

19. Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000; 47:277–290. PMID: 10802350.
20. Rose MA, Olivotto I, Cady B, Koufman C, Osteen R, Silver B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic results. Arch Surg. 1989; 124:153–157. PMID: 2916935.
21. Klinge U, Schumpelick V, Klosterhalfen B. Functional assessment and tissue response of short- and long-term absorbable surgical meshes. Biomaterials. 2001; 22:1415–1424. PMID: 11336316.

22. Kim HO, Hwang SI, Yom CK, Park YL, Bae WG. The use of absorbable surgical mesh after partial mastectomy for improving the cosmetic outcome. J Breast Cancer. 2009; 12:151–155.

23. Bourne RB, Bitar H, Andreae PR, Martin LM, Finlay JB, Marquis F. In-vivo comparison of four absorbable sutures: Vicryl, Dexon Plus, Maxon and PDS. Can J Surg. 1988; 31:43–45. PMID: 2827875.
24. Eom TI, Kim BS, Koo BY, Kim JW, Lim YA, Lee HH, et al. The use of a corrective procedure with Vicryl mesh for oncoplastic surgery of the breast. J Breast Cancer. 2009; 12:36–40.

25. Góes JC, Landecker A, Lyra EC, Henríquez LJ, Góes RS, Godoy PM. The application of mesh support in periareolar breast surgery: clinical and mammographic evaluation. Aesthetic Plast Surg. 2004; 28:268–274. PMID: 15666042.
26. Góes JC. Periareolar mammaplasty: double skin technique with application of polyglactine or mixed mesh. Plast Reconstr Surg. 1996; 97:959–968. PMID: 8618999.
27. Whitfield GA, Horan G, Irwin MS, Malata CM, Wishart GC, Wilson CB. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol. 2009; 90:141–147. PMID: 18977547.

28. Hill-Kayser CE, Vachani C, Hampshire MK, Di Lullo GA, Metz JM. Cosmetic outcomes and complications reported by patients having undergone breast-conserving treatment. Int J Radiat Oncol Biol Phys. 2012; 83:839–844. PMID: 22137022.

29. Livi L, Borghesi S, Saieva C, Meattini I, Rampini A, Petrucci A, et al. Radiotherapy timing in 4,820 patients with breast cancer: university of florence experience. Int J Radiat Oncol Biol Phys. 2009; 73:365–369. PMID: 18715726.

Figure 1
Cosmetic outcomes and sonographic findings. (A, D) Good cosmetic outcome. Sonography indicated echogenic materials (in a 51-year-old woman with infiltrating ductal carcinoma [IDC] at 20 months after operation). (B, E) Fair cosmetic result showed mild asymmetry and nipple deviation, but no contracture. Sonography indicated parenchymal distortion (in a 42-year-old woman with IDC at 24 months after operation). (C, F) Poor cosmetic result was expressed by severe contracture, asymmetry and pain. Sonography indicated seroma (in a 54-year-old woman with IDC at 18 months after operation).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download