Abstract
Purpose
This study aims to analyze the clinical-pathological characteristics of multifocal and multicentric breast cancer (MMBC) in Chinese women.
Methods
Sixty-seven cases with MMBC were randomly collected and reviewed at seven hospitals in representative districts of China during 1999 to 2008.
Results
The incidence of MMBC in breast cancer in China was 1.75%. Compared to those with unifocal breast cancer, women with MMBC were more likely to have larger tumor size, lymph node metastasis (59.70% vs. 45.62%) and stage III to IV (46.26% vs. 21.10%). The peak age at onset of MMBC was 40 to 49 years old and has been gradually increasing during 1999 to 2008. Most of the MMBC women were treated with surgery and adjuvant therapy.
Multifocal (MF) breast cancer and multicentric (MC) breast cancer are historically defined as two or more synchronous ipsilateral neoplasms, separated by benign tissue located with in the same quadrant or different quadrants of the breast [1-4]. The difference between MF breast cancer and MC breast cancer is based on the anatomic quadrant of the breast [5,6]. The multiple foci located in the same quadrant are labeled as MF, whereas MC disease is present in more than one quadrant. Some authors also distinguish MF breast cancer and MC breast cancer based on the assumption that MF breast cancer originates within the same duct collecting system (tumors in the same quadrant or less than or equal to 5 cm apart), whereas MC breast cancer originate in the different duct collecting systems (tumors in different quadrants or more than 5 cm apart), which means that MF breast cancer is monoclonal, while MC breast cancer is not [4,7-10]. At present, most investigators grouped MC and MF breast cancer together, as multifocal and multicentric breast cancer (MMBC), due to poorly defined anatomical borders between the quadrants and the difficulty in estimating the precise distance between tumors [4]. Another pragmatic reason is the fact that MF breast cancer (as presently defined) is more common than MC breast cancer [11].
With the widespread use of mammographic screening and improved sensitivity of imaging modalities, the detection of MMBC is likely to increase continuously [12]. The reported prevalence of MMBC varies widely in the literature, ranging from 9% to 75%, due to the lack of standardization in the gross examination and definition of MMBC [4,13-15]. Moreover, the extent of breast tissue sampling also plays an important role in the variability, as some investigators may sample the suspicious macroscopic areas, whereas the others would extensively use the whole mount sections [4].
In China, a large amount of work has been done to study the clinical-pathological features of MMBC in women. However, most studies have concentrated just on certain areas of China and did not represent the characteristics of MMBC in the whole women population in China. The aim of this study is to accumulate the clinical-pathological features of MMBC in Chinese women, which could represent the whole population in China during 1999 to 2008. In addition, the alteration of clinical-pathological features over the years and the shift of geographical distribution of MMBC in China can also be addressed from this retrospective cohort study.
The study is a multicenter retrospective research on primary breast cancer in Chinese women based in hospitals located in seven districts, including North China, Northeast China, Central China, South China, East China, Southwest China, and Northwest China. One representative hospital was selected from each of the seven areas according to the quality of the diagnosis and treatment of breast cancer. Every hospital was required to collect data randomly, which should include more than 50 cases or equal in any month from March to December during 1999 to 2008, which means a total of no less than 500 cases to be collected from each hospital over the years.
All cases were collected through the planned questionnaire. The data extracted from the cases include personal information, basic demographic characteristic, risk factors of breast cancer, diagnostic information (including clinical diagnosis and imaging diagnosis), treatment information (including surgical operation, chemotherapy, radiotherapy, and medical therapy), and clinical-pathological features (including pathological report and biomarker status). The quality of these data was controlled via clerk training, regular checks and sampling 5% to 10% case reports randomly to review. This study was approved by the Cancer Foundation of China Institutional Review Board (IRB approval No. is 20090806205). There were no anticipated risks for the enrolled patients; therefore, patient consent was not required for the study. All of the collected data were preserved on secure database, and only the research members have access to them.
The T stage of MMBC is determined by the diameter of the largest foci, according to the American Joint Committee on Cancer (AJCC) staging recommendations [16]. For MMBC, the following regulations should be kept when multiple tumors are different in histological or immunohistochemical features: the histological sub-type was in accordance with the index tumor when the tumors are different in histological sub-type, whereas the higher risk sub-type was recorded when the index tumor was a low risk sub-type; the grade accorded with the highest grade when the deposits differed in the grade; the receptor status was considered as receptor-positive when the foci were different in the receptor status; the human epidermal growth factor receptor 2 (HER2) status was in accordance with the higher score when the HER2 status differed in the immunohistochemical score [15].
All the statistical analyses were conducted with SPSS version 16.0 (SPSS Inc., Chicago, USA) to compare the clinical-pathological features of MMBC and those of unifocal breast cancer. Chi-square test was used to assess the differences in age, tumor size, estrogen receptor (ER) and progesterone receptor (PR) status between women with MMBC and unifocal breast cancer. For comparison of lymph node metastasis, stage, histological type, HER2 status, lymphovascular space invasion (LVSI), operation and adjuvant treatment, Fisher's exact test was used. The difference of the mean age in MMBC and unifocal breast cancer was evaluated by a t-test. All tests were two-sided, and p-values equal to or less than 0.05 were justified as statistically significant.
A total of 4,211 patients with breast cancer were collected, including 3,768 unifocal breast cancer women, 67 cases with MMBC and the other 367 cases whose data were not detailed to judge if they were MMBC cases or unifocal breast cancer cases. As a result, the 367 cases were excluded from the study. The data analysis showed 67 women (1.75%) of the collected 3,835 breast cancer cases were MMBC patients. The distribution of demographic, pathology, lymph node metastasis, receptor status, and treatments of these women is summarized in Table 1. The mean age at diagnosis of women with MMBC was 48.19±11.30 years, which was similar to that of women with unifocal breast cancer (48.77±10.51 years). Compared to those with unifocal breast cancer, women with MMBC were more likely to have larger tumor size, lymph node metastasis (59.70% vs. 45.62%) and stage III to IV (46.26% vs. 21.10%), while less likely to have ER positive (26.87% vs. 49.52%), PR positive (31.34% vs. 50.00%), HER2 receptor overexpressed (10.45% vs. 18.39%), LVSI (34.33% vs. 49.36%), and stage I to II (46.27% vs. 65.23%). There was no significant difference in the distribution of histological types between the MMBC and unifocal groups (p>0.05). Within the 67 women with MMBC, 57 (85.07%) had ductal carcinoma, 3 (4.48%) had lobular carcinoma and none had mixed ductal carcinoma.
The incidence of MMBC in breast cancer in different areas during 1999 to 2008 is summarized in Table 2. The total incidence of MMBC in breast cancer in 2001 was 3.18%, which was the highest in all the 10 years, while the lowest incidence was 0.40% occurring in 2008. No obvious regular change on the incidence of MMBC by year was observed. Of all the seven areas, Northwest China had the highest incidence (8.07%), and North China had the lowest, which was just 0.16%. In Northwest China, the incidence of MMBC in breast cancer was as high as 14.89% in 2001.
Figure 1 displays the distribution of MMBC patients' age at diagnosis. The peak age of onset of MMBC patients was 40 to 49 years old, which accounted for 41.79% (28/67), and the second largest group was the 50 to 59 years that accounted for 22.39% (15/67), and the 30 to 39 years group accounted for only 14.93% (10/67). In addition, none of the MMBC patients was younger than 19 years old, and only 4 women were over or equal to 70 years old. The trend of this age shift during 1999 to 2008 is detailed in Table 3. The percentages of MMBC women in both the 30 to 39 and 40 to 49 age groups have shown a downward trend during the 10 years. However, the 50 to 59 age group had an upward trend, which suggests that the peak age at onset of MMBC has been gradually increasing.
The treatment of MMBC women with different therapeutic strategies was showed in Table 4. Most of MMBC women received surgery and postoperative adjuvant therapy. Of the 67 MMBC patients, 21 (31.3%) had received surgery combined with chemotherapy, which was the dominant strategy. Surgery only, the second most popular treatment, was offered to 18 patients (26.9%). There were also 11 patients (16.42%) who were treated by surgery, chemotherapy, and radiotherapy. Within 4 patients (5.97%) who did not receive surgery, 3 were located in South China and 1 in East China. Among the 63 surgical patients, 58 (92.06%) women underwent mastectomy, with only 1 (1.59%) located in Northwest China, where there were 38 MMBC patients undertaking breast-conserving surgery (Table 5). The conservative surgery has been conducted in 2006, and none of MMBC patients have received this surgery during 1999 to 2005 (Table 6).
This is the first study that investigates the clinical-pathological characteristics of MMBC in women covering most areas of China.
The key characteristics of MMBC are established independent prognostic factors, including age at diagnosis, the largest tumor diameter, pathologic nodal status, histopathological grading, histological type, hormone receptor status, HER2 status, and LVSI [17]. Therefore, improving the cognition of clinical and pathological features of MMBC contributes to selection of appropriate treatment. Our study addresses that in China, compared with unifocal women, MMBC women were more likely to be younger, have axillary nodal involvement, larger tumor size and higher stage. The receptor status of the two groups was also significantly different, albeit the histological types were similar. More recent reports in the world have also described the features of the women with MMBC [15,18]. Yerushalmi et al. [18] reported that the MMBC group was more likely to have axillary nodal involvement, LVSI and invasive lobular histology, compared to that of the unifocal group, while the two groups were similar in terms of age, tumor size and grade. More recently, Rezo et al. [15] found that women with MF breast cancer were more likely to be less than 50 years old, have nodal involvement, a larger tumor size and have LVSI, when compared to women with unifocal breast cancer, and there were no significant difference in histological sub-type, grade, or hormone receptor status between these two groups. Of note, a consistent finding of these studies, including ours is that MMBC women have a higher frequency of axillary node involvement than women with unifocal cancer, and some other series have also reported a higher risk of pathological axillary nodal involvement for women with MMBC [19-24], which suggest that MMBC is more biologically aggressive than unifocal breast cancer.
Our reported incidence of MMBC in breast cancer is 1.75%, significantly lower than the data (9-75%) reported by the studies in other countries [4,13-15], which may reflect the differences in the availability of healthcare resources, such as the sensitivity of mammography, ultrasound and magnetic resonance imaging for detecting multiple malignant foci and the level of pathological diagnosis, in addition to the genetic background, environmental and social economic factors, as well as life style and eating habits. Of note, the incidence of MMBC in Northwest China is as high as 8.07%, which is much higher than the data of other areas in China, but is close to other countries. The possible correlations are yet to be discussed.
This study also identified aging trend of Chinese women with MMBC, which was similar to Western women. The reasons involved may be due to more Westernized lifestyle of Chinese people with globalization and the increasing aging population in China. It is noteworthy that the result would help to guide the screening of target populations.
The treatment for MMBC has changed over the years, especially the surgical treatment. Investigators used to consider that MMBC should be treated with mastectomy due to the documented unacceptable high rates of local recurrence in MMBC women treated with breast conservation. In early trials, the local regional recurrence in MMBC women with breast conservation ranged from 23% to 40% at a 5-year follow-up [24-27]. These studies concluded that the risk of local recurrence was too high to be accepted, and hence, mastectomy was recommended for MMBC population. In contrast, more recent studies have proposed breast-conserving surgery to MMBC women as long as the principles of negative margins, appropriate radiotherapy and acceptable cosmesis are met [12,28-30]. These researchers reported a range of local regional recurrence from 3% to 5.1% at a 6-year follow-up in MMBC women with breast conservation, which was not different from the patients with unifocal tumor, and they did not find any apparent differences in terms of disease-free survival or cosmetic result in comparison of MMBC patients to the women with unifocal breast cancer after breast-conserving treatment. Therefore, breast-conserving surgery is safe and effective for MMBC patients. In our study, most of MMBC patients underwent mastectomy and only one received breast-conserving treatment, the possible reason could be that the breast of Chinese women is much smaller than that of the Western population; therefore, it is very difficult to reach an ideal cosmetic result if enough breast tissue is surgically excised from Chinese MMBC patient in order to achieve the negative margin. Just because of this, mastectomy is still recommended to Chinese MMBC patients instead of breast conservative surgery.
Most of MMBC patients in our study received surgery and postoperative adjuvant therapy, such as chemotherapy, endocrine therapy and radiotherapy, which are consistent with the treatment of unifocal breast cancer. When it comes to breast cancer, surgery combined with integrated adjuvant treatment is obviously better than surgery alone; therefore, appropriate adjuvant therapy after surgery has already become an international consensus, so as to MMBC.
This is a retrospective study, and therefore, some limitations need to be addressed. Firstly, we failed to collect the follow-up data of the patients. Without the follow-up, we cannot compare the locoregional control, disease-free survival, and overall survival between MMBC patients and those with unifocal disease. Therefore, we cannot assess whether MMBC patients have an inferior outcome in China. We also failed to analyze independent prognostic factors of Chinese women with MMBC due to the lack of follow-up data. Secondly, part of the data we have collected are short of accuracy, which might not comprehensively reflect the characteristics of Chinese women with MMBC. Despite the above limitations, we still reckon that these data are of important clinical interest because only a few papers with limited number of cases have been reported on this subject in China up to now.
In conclusion, in China, the incidence of MMBC in breast cancer is significantly lower than that in Western countries. The peak age at onset of MMBC is 40 to 49 years old and has been gradually increasing during 1999 to 2008. Compared to unifocal breast cancer, MMBC is biologically more aggressive. The main treatment for MMBC is surgery in combination with postoperative adjuvant therapy; of note, in China, most MMBC women undergo mastectomy instead of breast conservation surgery, which has not been extensively received so far.
Figures and Tables
Figure 1
The age proportion of multifocal and multicentric breast cancer in China during 1999 to 2008.
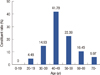
Table 1
Comparision of patient demographics and tumor characteristic between MMBC and unifocal tumors during 1999 to 2008

MMBC=multifocal and multicentric breast cancer; LD=diameter of the largest foci; LNM=lymph node metastasis; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; LVSI=lymphovascular space invasion.
*Unknown cases are not included in the chi-square test, Fisher's exact test, and t-test; †Chi-square test; ‡t-test; §Fisher's exact test.
ACKNOWLEDGEMENTS
We thank the local investigators from Beijing, Liaoning (Shenyang), Hunan (Changsha), Guangdong (Guangzhou), Zhejiang (Hangzhou), Shannxi (Xian), and Sichuan (Chengdu) for data collection and assisting us complete the project successfully.
References
1. Lagios MD, Westdahl PR, Rose MR. The concept and implications of multicentricity in breast carcinoma. Pathol Annu. 1981. 16:83–102.
2. Oh JL, Dryden MJ, Woodward WA, Yu TK, Tereffe W, Strom EA, et al. Locoregional control of clinically diagnosed multifocal or multicentric breast cancer after neoadjuvant chemotherapy and locoregional therapy. J Clin Oncol. 2006. 24:4971–4975.

3. Boyages J, Jayasinghe UW, Coombs N. Multifocal breast cancer and survival: each focus does matter particularly for larger tumours. Eur J Cancer. 2010. 46:1990–1996.

4. Bozzini A, Renne G, Meneghetti L, Bandi G, Santos G, Vento AR, et al. Sensitivity of imaging for multifocal-multicentric breast carcinoma. BMC Cancer. 2008. 8:275.

5. Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985. 56:979–990.

6. Fisher ER, Sass R, Fisher B, Gregorio R, Brown R, Wickerham L. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer. 1986. 57:1717–1724.

7. Noguchi S, Aihara T, Koyama H, Motomura K, Inaji H, Imaoka S. Discrimination between multicentric and multifocal carcinomas of the breast through clonal analysis. Cancer. 1994. 74:872–877.

8. Teixeira MR, Pandis N, Bardi G, Andersen JA, Bøhler PJ, Qvist H, et al. Discrimination between multicentric and multifocal breast carcinoma by cytogenetic investigation of macroscopically distinct ipsilateral lesions. Genes Chromosomes Cancer. 1997. 18:170–174.

9. Tsuda H, Hirohashi S. Identification of multiple breast cancers of multicentric origin by histological observations and distribution of allele loss on chromosome 16q. Cancer Res. 1995. 55:3395–3398.
10. Wa CV, DeVries S, Chen YY, Waldman FM, Hwang ES. Clinical application of array-based comparative genomic hybridization to define the relationship between multiple synchronous tumors. Mod Pathol. 2005. 18:591–597.

11. Khan SA. The many questions that surround multicentric and multifocal breast cancer. Breast J. 2010. 16:219–221.

12. Bauman L, Barth RJ, Rosenkranz KM. Breast conservation in women with multifocal-multicentric breast cancer: is it feasible? Ann Surg Oncol. 2010. 17:Suppl 3. 325–329.

13. Fisher ER, Gregorio R, Redmond C, Vellios F, Sommers SC, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol no. 4). I. Observations concerning the multicentricity of mammary cancer. Cancer. 1975. 35:247–254.

14. Jain S, Rezo A, Shadbolt B, Dahlstrom JE. Synchronous multiple ipsilateral breast cancers: implications for patient management. Pathology. 2009. 41:57–67.

15. Rezo A, Dahlstrom J, Shadbolt B, Rodins K, Zhang Y, Davis AJ, et al. Tumor size and survival in multicentric and multifocal breast cancer. Breast. 2011. 20:259–263.

16. American Joint Committee on Cancer. American Joint Committee on Cancer. Breast. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer;223–240.
17. Weissenbacher TM, Zschage M, Janni W, Jeschke U, Dimpfl T, Mayr D, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010. 122:27–34.

18. Yerushalmi R, Kennecke H, Woods R, Olivotto IA, Speers C, Gelmon KA. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat. 2009. 117:365–370.

19. Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005. 23:7497–7502.

20. Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Outlaw ED, Strom EA, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005. 103:689–695.

21. Andea AA, Bouwman D, Wallis T, Visscher DW. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer. 2004. 100:20–27.

22. Andea AA, Wallis T, Newman LA, Bouwman D, Dey J, Visscher DW. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002. 94:1383–1390.

23. Chua B, Ung O, Taylor R, Boyages J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg. 2001. 71:723–728.

24. Fowble B, Yeh IT, Schultz DJ, Solin LJ, Rosato EF, Jardines L, et al. The role of mastectomy in patients with stage I-II breast cancer presenting with gross multifocal or multicentric disease or diffuse microcalcifications. Int J Radiat Oncol Biol Phys. 1993. 27:567–573.

25. Leopold KA, Recht A, Schnitt SJ, Connolly JL, Rose MA, Silver B, et al. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys. 1989. 16:11–16.

26. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990. 212:38–44.

27. Wilson LD, Beinfield M, McKhann CF, Haffty BG. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer. 1993. 72:137–142.

28. Okumura S, Mitsumori M, Yamauchi C, Kawamura S, Oya N, Nagata Y, et al. Feasibility of breast-conserving therapy for macroscopically multiple ipsilateral breast cancer. Int J Radiat Oncol Biol Phys. 2004. 59:146–151.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download