Abstract
Purpose
We investigated the association between dietary intake of citrus fruits and breast cancer risk.
Methods
The PubMed and EMBASE were searched for relevant articles on diet and breast cancer up to January 2012. All of the epidemiological studies that assessed dietary intake of citrus fruits and presented risk estimates of the association between citrus fruits intake and risk of breast cancer were reviewed. Multivariable-adjusted odds ratios (OR) and associated 95% confidence intervals (CI) for highest versus lowest intake of dietary citrus fruits level were extracted. Overall summary OR was calculated by using a fixed-effect meta-analysis.
Results
Six case-control studies out of five articles were eligible. Overall summary OR showed a 10% reduction in risk of breast cancer associated with high intake of citrus fruits (summary OR, 0.90; 95% CI, 0.85-0.96; p<0.001); results were consistent across the studies (I2=0). Visual inspection of the results did not suggest a publication bias.
Breast cancer was one of the most common cancers in 2008 and overtook stomach cancer as the cancer with the highest incidence in Korean women in 2001 [1]. The incidence rate of breast cancer has been increasing globally [2], and that of Korean women has been rising gradually in the 2000s [1].
Diet and nutrition have been emphasized as a modifiable risk factor for breast cancer, while most of the other factors, i.e., reproductive history, lactation, menstrual history, adult-attained height and obesity, are generally difficult to modify [3]. The 2007 World Cancer Research Fund report concluded that fruits in general likely protect against cancers of the mouth, pharynx, and larynx, and those of the esophagus, lungs, and stomach. Vegetable and fruits are low in energy and contain various micronutrients which act as markers for consumption [3]. The theory that vegetables and fruits protect against some cancers is supported by evidence from studies on foods containing carotenoids [4], β-carotene [5], lycopene [6], folate [7], vitamin C [8], vitamin E [9], B-vitamin pyridoxine (vitamin B6) [10], selenium [11], and quercetin [12].
Recently, a systematic review reported on the protective effects of high citrus fruit intake on stomach cancer risks [13]. Another systematic review on citrus fruits has followed, reporting on an inverse association with citrus fruit ingestion and pancreatic cancer risk, although the effect was limited due to weak study design [14]. Citrus fruits are complex sources of β-cryptoxanthin (carotenoid), β-carotene, folate, vitamin C, and Quercetin (flavonoid) [4]. They are fruits commonly eaten including oranges, tangerines, grapefruits, lemons, and limes [4]. Taking the above into consideration, we have conducted a systematic review and meta-analysis to explore the hypothesis that dietary intake of citrus fruits may be associated with a reduced risk of breast cancer.
An electronic literature search was conducted in PubMed (U.S. National Library of Medicine, Bethesda, USA) and EMBASE (Reed Elsevier PLS, Amsterdam, The Netherlands) to identify human adult studies written in the English language and published up to January 2012 that included the following keywords or phrases: breast, breast neoplasms, fruit, citrus, diet, dietary, prevention and control, etiology, epidemiology, humans, and adult. The search terms used were: ("Breast Neoplasms/diet therapy"[Majr] OR "Breast Neoplasms/epidemiology"[Majr] OR "Breast Neoplasms/etiology"[Majr] OR "Breast Neoplasms/prevention and control"[Majr]) AND ("Fruit" [Mesh] OR "Citrus"[Mesh]) AND ("humans"[MeSH Terms] AND Comparative Study[ptyp] AND "adult"[MeSH Terms]) for PubMed; and breast AND [(neoplasm) OR (cancer)] AND [(FRUIT) OR (CITRUS)] AND [(PREVENTION) OR (RISK) OR (ETIOLOGY)] for EMBASE. In addition, we reviewed the references cited in the full-text articles and in the relevant review articles or meta-analyses identified in the search.
We applied the following inclusion criteria [13]: 1) epidemiological studies including case-control or cohort studies; 2) human adult participants; and 3) studies addressing the association between fruit intake and breast cancer. The full-text articles of all references selected by the inclusion criteria were collected. The following exclusion criteria were applied to the full-text articles including potential references listed by hand-search: 1) no original data, that is, reviews, meta-analysis; 2) studies not measuring the intake of citrus fruit or citrus juice at the individual level; and 3) studies not reporting the standard error (SE) of the associated measure of association. Two independent reviewers read the abstracts or full-text articles to assess the eligibility in a standardized manner. Disagreements between reviewers were resolved by consensus.
The following information was extracted from all of the eligible studies: study design, country of origin, years of enrollment, sampling frame, number of participants, range of age, kinds of citrus fruits, level of comparison, and potential confounding variables that had been adjusted for. From the eligible studies that met the inclusion criteria, estimates of the odds ratio (OR)/relative risk (RR), and their associated 95% confidence intervals (CIs), were calculated for the data relating to the intake of citrus fruits. If separate articles from the same study were published, the article containing the more detailed information on the case and control was selected for inclusion.
Using general variance-based methods, study-specific OR/RR and 95% CIs for the highest versus the lowest intake of citrus fruits level were extracted from each article. For all studies, the reported OR/RR estimate was adjusted for age. SE=[ln (OR/RR upper limit)-ln (OR/RR lower limit)]/2×1.96. Where OR were given by menopause status (e.g., premenopausal or postmenopausal) [15]; separate estimates were obtained by fixed-effects meta-analysis.
Heterogeneity was tested with a chi-square test and measured by using the I2 statistic. The I2 describes the percentage of total variation across studies because of study differences rather than chance. A fixed-effect model was used to calculate the summary OR and its 95% CI when substantial heterogeneity was not observed. Each study's estimate and SE was used to produce a forest plot that gave a pooled estimate. In an attempt to detect publication bias, we visually examined asymmetry in the Begg's funnel plot. We used Cochrane Collaboration software RevMan 5.0 (Oxford, UK) to analyze the extracted data using fixed effects model analysis.
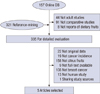
The computerized search yielded 157 articles, and 321 articles that had been identified from the citations were added. Of the 335 articles that were obtained for full-text review, we excluded 330 articles based on the exclusion criteria (Figure 1). In particular, the results of Li et al. [16] were replaced by those of Shannon et al. [17], as it shared the same database. Finally, 5 articles were included in the meta-analysis [15,17-20], including one article that reported on two ORs from two study groups classified by menopausal status [15].
The six studies included in the final analysis had 8,393 participants: 3,789 cases and 4,705 controls. Some details of the selected studies are shown in Table 1. All articles were published in English. Three studies were conducted among the residents of China [17-19], two studies were from the U.S. [15], and one study was from Australia [20]. Five of the studies recruited participants in the 2000s and one in the 1990s.
Four studies adjusted for age [15,17,19], and another four adjusted for total energy intake [15,18,19]. Common adjustment factors of three studies included age at menarche and family history of breast cancer [18-20]. Studies differed in factors considered as potential confounders (Table 1). The potential confounders were education, breast-feeding, parity, age at first live birth, history of benign breast disease, body mass index (BMI), waist-to-hip ratio, physical activity, and total fruit and vegetable intake.
In all of the studies, intake of citrus fruits was part of a broader dietary assessment, and the relationship between citrus fruit intake and breast cancer was not a primary hypothesis. The adjusted OR for the highest category for citrus fruit intake varied considerably, with the ORs ranging from 0.68 to 1.1. One study reached the usual threshold p-value of 0.05 [19].
There was no significant heterogeneity among the study results (I2=0, p=0.48). The overall summary OR using the fixed effect model showed a 10%, statistically significant reduction in the risk of breast cancer for the highest intake group when compared to the lowest (summary OR, 0.90; 95% CI, 0.85-0.96) (Figure 2).
No publication bias was observed in the selected studies. Visualization of Begg's funnel plot was symmetrical (Figure 3).
The results indicate that a higher intake of citrus fruits may decrease breast cancer risks. To the best of our knowledge, this is the first systemic review and meta-analysis of breast cancer and citrus fruit intake. When the latest World Cancer Research Fund report was published in 2007, there was no convincing evidence for individual foods and nutrients modifying the risk of breast cancer except for alcohol consumption. It was judged that the evidence was too limited to reach a conclusion on the effect of fruits on breast cancer risk [3]. Up to now, only one [18] of the six studies included in our analyses [15,17-20] was published subsequent to the 2007 World Cancer Research Fund report. It showed a nonsignificant decreased risk for the highest intake group when compared to the lowest intake group, with an effect estimate of 0.73 (95% CI, 0.50-1.06) and a p-value for trend of 0.17 [18].
The results of this quantitative meta-analysis could be supported by the finding of the lowest age-standardized incidence rate of breast cancer in Korea occurring in the population of Jeju who simultaneously consumed the highest amount of tangerines in Korea [21-23]. The previous two quantitative meta-analyses reporting a protective effect of high citrus fruit intake in stomach and pancreas cancer risk, respectively [13,14], also seem to exist in the same context [24].
The following study limitations are considered. Firstly, although the selected studies for this meta-analysis were homogeneous with zero I2 (p=0.48), the data were supplementary, as from observational studies originally designed to test other hypotheses. Because of a diversity of study design among the studies we analyzed as well as the existence of potential biases, it may be problematic to interpret the pooled results as a simple summary. In addition, although all the selected studies had collected information on factors considered as potential confounders, such as demographic characteristics, gynecologic and reproductive history, medication history, family history of breast cancer, smoking, BMI, physical activity, and energy intake, the factors adjusted for in the individual analyses of citrus fruit intake were not identical.
Secondly, the apparent association of this pooled result is restricted to the case-control studies. As retrospective studies are considered as more prone to overestimating the exposure effect due to recall and selection bias, it is possible that any true ORs are likely smaller than as shown in this analysis.
Thirdly, measurement error in a food frequency questionnaire (FFQ), such as inability to accurately capture the intake of all citrus fruits, should be considered. It has been shown that frequency of intake explains most of the variations in intake. While the FFQ may be an adequate, albeit imperfect, instrument for measuring relative fruit and vegetable intake, the nondifferential misclassification errors observed in the FFQ likely attenuate the estimates toward the null [25]. Consequently, there is another possibility that any true ORs are likely greater than as shown in this analysis. Lastly, as the cutoff points for citrus fruit intake vary across studies, there is uncertainty on the optimal amount of citrus fruit intake for the prevention of breast cancer.
Environmental factors are important in the progression of the disease, although between 4% and 9% of breast cancer cases are hereditary with mutations in either the BRCA1 or BRCA2 gene [26]. The risk of breast cancer of migrants from areas of low risk to areas of high risk increased by as much as six-fold within one to two generations, likely due to changes in lifestyle [27]. Early life events, including food and nutrition, as well as other life events which affect the number of menstrual cycles have an effect on lifetime exposure to estrogen. Food and nutrition influence the age of breast development and menopause, with high-energy diets promoting earlier puberty and late menopause, and low-energy diets delaying puberty and advancing menopause [3].
Citrus fruits contain a complex mixture of constituents, all of which may also contribute to any effect [3]. While it is not possible to ascertain which constituents in citrus fruits play a significant role in breast cancer, relevant mechanisms have been available. Vitamin C traps free radicals and reactive oxygen molecules, and regenerates other antioxidant vitamins [28]. Vitamin C also inhibits the formation of carcinogens which attack DNA to mutagenic changes [29]. Two citrus flavonoids, hesperetin and naringenin, are found in orange and grapefruit, respectively. An experimental study has shown that citrus flavonoids are effective inhibitors of human breast cancer cell proliferation in vitro, especially when paired with quercetin, widely distributed in other foods [30]. Beta-carotene and other carotenoid antioxidants are also found in citrus fruits.
In summary, pooled results from observational studies show an inverse association between citrus fruit intake and the risk of breast cancer. This statistical evidence helps to generate a new hypothesis of citrus fruits modifying the risks of breast cancer. However, the limitations as outlined above may present questions on showing a true association. As such, the need for well-designed prospective observational and intervention studies is highlighted by this study to clarify the role of citrus fruit intake and breast cancer.
Figures and Tables
 | Figure 2Summary estimates of the association between citrus fruits intake and breast cancer risks sorted by effect estimate.
CI=confidence interval; df=degree of freedom; chi2=chi-square statistic; I2=the percentage of total variation across studies that is due to heterogeneity rather than change; fixed=using fixed-effect model.
|
References
1. National Cancer Center. Ministry of Health and Welfare. Cancer Facts & Figures 2011 in the Republic of Korea. 2011. Goyang: National Cancer Center, Ministry of Health and Welfare;7.
2. Parkin DM, Fernández LM. Use of statistics to assess the global burden of breast cancer. Breast J. 2006. 12:Suppl 1. S70–S80.

3. World Cancer Research Fund. American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2007. Washington, DC: WCRF/AICR;289–295.
4. Shekelle RB, Lepper M, Liu S, Maliza C, Raynor WJ Jr, Rossof AH, et al. Dietary vitamin A and risk of cancer in the Western Electric study. Lancet. 1981. 2:1185–1190.

5. Franceschi S, Bidoli E, Negri E, Zambon P, Talamini R, Ruol A, et al. Role of macronutrients, vitamins and minerals in the aetiology of squamous-cell carcinoma of the oesophagus. Int J Cancer. 2000. 86:626–631.

6. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002. 94:391–398.

7. Larsson SC, Håkansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006. 98:407–413.

8. Eichholzer M, Stähelin HB, Lüdin E, Bernasconi F. Smoking, plasma vitamins C, E, retinol, and carotene, and fatal prostate cancer: seventeen-year follow-up of the prospective basel study. Prostate. 1999. 38:189–198.

9. Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, et al. Serum vitamin E and risk of cancer among Finnish men during a 10-year follow-up. Am J Epidemiol. 1988. 127:28–41.

10. Zhang SM, Moore SC, Lin J, Cook NR, Manson JE, Lee IM, et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. 2006. 163:108–115.

11. Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, et al. Prediagnostic serum selenium and risk of cancer. Lancet. 1983. 2:130–134.

12. Garcia-Closas R, Agudo A, Gonzalez CA, Riboli E. Intake of specific carotenoids and flavonoids and the risk of lung cancer in women in Barcelona, Spain. Nutr Cancer. 1998. 32:154–158.

13. Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008. 11:23–32.

14. Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Pancreas. 2009. 38:168–174.
15. Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, Teitelbaum SL, et al. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev. 2004. 13:1485–1494.
16. Li W, Ray RM, Lampe JW, Lin MG, Gao DL, Wu C, et al. Dietary and other risk factors in women having fibrocystic breast conditions with and without concurrent breast cancer: a nested case-control study in Shanghai, China. Int J Cancer. 2005. 115:981–993.

17. Shannon J, Ray R, Wu C, Nelson Z, Gao DL, Li W, et al. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005. 14:81–90.
18. Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, Lin FY. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009. 125:181–188.

19. Malin AS, Qi D, Shu XO, Gao YT, Friedmann JM, Jin F, et al. Intake of fruits, vegetables and selected micronutrients in relation to the risk of breast cancer. Int J Cancer. 2003. 105:413–418.

20. Ingram DM, Nottage E, Roberts T. The role of diet in the development of breast cancer: a case-control study of patients with breast cancer, benign epithelial hyperplasia and fibrocystic disease of the breast. Br J Cancer. 1991. 64:187–191.

21. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer Incidence in Five Continents. 2007. Vol. IX. Lyon: IARC.
22. Community of Population-based Regional Cancer Registries in Korea. An estimation of the national cancer incidence in Korea for 2000-2002 using the databases of 8 population-based regional cancer registries. J Prev Med Public Health. 2008. 41:380–386.
23. Korea Centers for Disease Control & Prevention. 2001 Report of Korea National Health and Nutrition Survey. Accessed February 15th, 2012. http://knhanes.cdc.go.kr.
24. Bae JM. Explaining cancer incidence in the Jejudo population. J Prev Med Public Health. 2009. 42:67–72.

25. Kipnis V, Midthune D, Freedman LS, Bingham S, Schatzkin A, Subar A, et al. Empirical evidence of correlated biases in dietary assessment instruments and its implications. Am J Epidemiol. 2001. 153:394–403.

26. Blackwood MA, Weber BL. BRCA1 and BRCA2: from molecular genetics to clinical medicine. J Clin Oncol. 1998. 16:1969–1977.

27. Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993. 85:1819–1827.

28. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003. 22:18–35.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download