Abstract
Purpose
Exemestane has shown good efficacy and tolerability in postmenopausal women with hormone receptor-positive metastatic breast cancer. However, clinical outcomes in Korean patients have not yet been reported.
Methods
Data on 112 postmenopausal women with metastatic breast cancer were obtained retrospectively. Clinicopathological characteristics and treatment history were extracted from medical records. All patients received 25 mg exemestane daily until objective disease progression. Progression-free survival (PFS) was the primary endpoint, and secondary endpoints were overall survival (OS), objective response rate (ORR), and clinical benefit rate (CBR=complete response+partial response+stable disease for 6 months).
Results
The median age of the subjects was 55 years (range, 28-76 years). Exemestane treatment resulted in a median PFS of 5.7 months (95% confidence interval [CI], 4.4-7.0 months) and median OS of 21.9 months (95% CI, 13.6-30.3 months). ORR was 6.4% and CBR was 46.4% for the 110 patients with evaluable lesions. Symptomatic visceral disease was independently associated with shorter PFS (hazard ratio, 3.611; 95% CI, 1.904-6.848; p<0.001), compared with bone-dominant disease in a multivariate analysis of PFS after adjusting for age, hormone receptor, human epidermal growth factor receptor 2, Ki-67 status, dominant metastasis site, and sensitivity to nonsteroidal aromatase inhibitor (AI) treatment. Sensitivity to previous nonsteroidal AI treatment was not associated with PFS, suggesting no cross-resistance between exemestane and nonsteroidal AIs.
Endocrine therapy is a well-established standard treatment in postmenopausal women with hormone receptor-positive metastatic breast cancer (MBC) that has shown good efficacy and tolerability [1]. Currently available options for MBC include third-generation nonsteroidal aromatase inhibitors (anastrozole and letrozole), selective estrogen receptor (ER) modulators (tamoxifen and toremifene), progestin (megestrol acetate), androgen (fluoxymesterone), high-dose estrogen (ethinyl estradiol), a selective ER down-regulator (fulvestrant), and a steroidal aromatase inhibitor (AI) (exemestane) [2].
Exemestane is similar in structure to androstenedione, a natural aromatase substrate. Exemestane irreversibly binds to the aromatase active site to permanently inactivate the enzyme [3]. Its efficacy as a first-line hormone treatment in MBC has been established in a randomized phase III trial that compared exemestane to tamoxifen, although the study failed to demonstrate superiority of exemestane [4]. A phase III trial reported a better response rate and overall survival for patients taking exemestane than for those taking megestrol acetate after tamoxifen failure [5]. The efficacy and toxicity profile of exemestane in the Evaluation of Faslodex versus Exemestane Clinical Trial (EFECT) study was comparable to that of fulvestrant in patients whose disease had progressed after treatment with a nonsteroidal AI (NSAI) [6].
However, large trials evaluating exemestane have been conducted mainly in Western populations, and clinical outcomes in Korean patients have not been reported. Korea's national medical insurance covers exemestane for postmenopausal women who have progressed on tamoxifen or NSAIs. Therefore, we investigated the efficacy of exemestane as a subsequent treatment after NSAI failure in Korean women in this single-center study.
Eligible patients were identified from the breast cancer database of Seoul National University Hospital, Seoul, Republic of Korea. All patients were postmenopausal women with metastatic or recurred disease that was ER-positive or progesterone receptor (PR)-positive, and had progressed after NSAI treatment or diseases had recurred during or after adjuvant NSAI treatment. Menopause was defined as follows: prior bilateral oophorectomy, age >60 years and age less than 60 years and amenorrheic for 12 or more months in the absence of chemotherapy, tamoxifen, toremifene, or ovarian suppression and follicle-stimulating hormone (FSH) and estradiol in the postmenopausal range [2]. A total of 118 patients were identified using these criteria; however, six patients were excluded because they were lost to follow-up before the first-response evaluation. This protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1110-021-380) and was exempted from the requirement to obtain informed consent.
The medical records of enrolled patients were reviewed to collect demographic, clinical, and pathology data. Treatment history, dominant site of metastasis, and clinical outcomes were extracted from the medical records. We summarized the ER, PR, human epidermal growth factor receptor 2 (HER2), and Ki-67 status as determined by immunohistochemical (IHC) staining, and HER2 gene amplification status as determined by fluorescent in situ hybridization (FISH). Tumors with an IHC score of 3+ or showing unequivocal amplification by FISH (HER2/CEP17 ratio >2.0) were considered HER2-positive [7]. Tumors with negative FISH results, or with IHC scores of 0- 2+ in the absence of FISH results, were considered HER2-negative. Ki-67 was considered high if IHC showed that ≥14% cells were positive [8]. Radiological responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Tumors were defined as NSAI-sensitive if the patient had a complete response, partial response, or stable disease for at least 6 months during previous NSAI treatment, or disease recurred 2 years after initiating adjuvant NSAI treatment. All other tumors including those that recurred within 2 years of adjuvant NSAI treatment were categorized as NSAI-resistant. Progression-free survival (PFS) was measured from the first day of exemestane treatment until radiological or clinical disease progression. Overall survival (OS) was calculated from the first day of exemestane treatment to patient death or the last date of follow-up. Objective response rate (ORR) was defined as the proportion of patients with the best response of either a complete or partial response. The clinical benefit rate (CBR) was defined as the proportion of patients achieving a best overall response of complete response, partial response, or stable disease for at least 6 months.
All patients received 25 mg exemestane daily until objective disease progression or other event that required treatment termination. Patients were seen regularly during the treatment period by their medical oncologists and underwent physical examinations and laboratory testing, including complete blood cell counts and a chemistry panel. Treatment response was evaluated using the appropriate imaging modalities every 2 months for the first 6 months and every 3 months thereafter or whenever there was clinical suspicion of disease progression.
Categorical variables were compared with the Pearson's chi-square test or Fisher's exact test, as appropriate. Median OS and PFS were calculated using the Kaplan-Meier method. Groups were compared by the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. Two-sided p-values <0.05 were considered significant. All analyses were performed using the PASW version 18.0 software package (SPSS Inc., Chicago, USA).
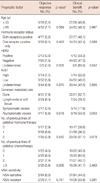
A total of 112 postmenopausal women with metastatic, hormone receptor-positive breast cancer were included in this analysis. Clinicopathological characteristics and previous treatment history are summarized in Table 1. The mean age at diagnosis of metastatic or recurred disease was 53.6±9.9 years (median, 54.5 years). Of the 112 patients, 78 (69.6%) were ER and PR positive, 28 (25.0%) were ER-positive/PR-negative, and six (5.4%) were ER-negative/PR-positive. HER2 status was known for 107 patients, and 12 patients (10.7%) were HER2-positive. Ki-67 was determined in 67 patients; 14 (12.5%) were high, and 53 (47.3%) were low. Fifty-two patients (46.4%) had bone-dominant disease at the initiation of exemestane treatment, and 25 patients (22.3%) were lymph node- or soft tissue-dominant. Of the 35 patients (31.3%) who had viscera-dominant disease, 17 (48.6%) were symptomatic because of extensive visceral metastases, and 18 (51.4%) had small visceral metastases without symptoms.
All patients had a history of hormone therapy including treatment with selective estrogen receptor modulators and NSAIs. Among the 94 patients with recurred disease, 66 (70.2%) underwent adjuvant hormone therapy after surgery. For metastatic disease, 18 patients (16.1%) used exemestane as first-line treatment after failure of adjuvant NSAI. Fifty-nine patients (52.7%) used exemestane as a second-line hormonal agent for metastatic disease, 31 (27.7%) as third-line treatment, and four (3.6%) as fourth-line treatment. The median number of palliative chemotherapy regimens prior to exemestane treatment was two, and 32% of the patients (36/112) had received three or more prior chemotherapy treatments. About 35% of the patients (41/112) underwent palliative radiotherapy before exemestane treatment.
The median follow-up for the 112 patients treated with exemestane was 53.5 months, and the median duration of treatment was 5.6 months. Seventeen patients (15.2%) were still receiving exemestane at the data cutoff point. The most common reason for discontinuing exemestane was disease progression (96.8%), followed by patient refusal (2.1%), and drug toxicity (arthralgia grade 3, 1.1%).
The median PFS and OS were 5.7 months (95% CI, 4.4-7.0 months) (Figure 1) and 21.9 months (95% CI, 13.6-30.3 months), respectively. Among the 110 patients with evaluable lesions, seven (6.4%) showed an objective response to exemestane, and the CBR was 46.4% (Table 2). The median PFS did not differ among patients, whether they received exemestane as first-line (5.7 months), second-line (4.8 months), or third- or later-line palliative hormone therapy (6.2 months, p=0.745). Similarly, the median PFS did not differ significantly between the NSAI-sensitive (5.5 months) and NSAI-resistant groups (11.2 months, p=0.164). Results of a subgroup analysis revealed that the CBR for patients with symptomatic visceral disease (17.6%) was significantly lower than that of patients with bone-dominant disease (62.7%), lymph node- or soft tissue-dominant disease (29.2%), or asymptomatic visceral disease (50.0%, p=0.003) (Table 3). A multivariate analysis was performed to determine whether age, hormone receptor status, HER2 status, Ki-67, dominant metastatic site, or NSAI sensitivity predicted PFS. The results showed that only symptomatic visceral metastasis was associated with a shorter PFS (hazard ratio, 3.611; 95% CI, 1.904-6.848; p<0.001). NSAI sensitivity was not significantly associated with PFS (Table 4).
Assessment of toxicity was limited due to the retrospective nature of this study. Most adverse events were mild, except for grade 3 arthralgia and nausea toxicity in two cases. The three most frequently observed adverse events were arthralgia (3.6%), fatigue (3.6%), and myalgia (2.7%). Information about bone health was available in 34 patients (30.4%); three (8.8%) patients experienced new-onset or worsening osteoporosis while using exemestane, which were defined by National Cancer Institute-Common Toxicity Criteria (version 4.0).
We evaluated clinical outcomes of Korean postmenopausal women with hormone receptor-positive MBC treated with exemestane. Exemestane treatment resulted in a median PFS of 5.7 months and median OS of 21.9 months (ORR, 6.4%; CBR, 46.4%). The patients in this study had been heavily pretreated, as they had received a median of two lines of chemotherapy and two lines of hormone therapy. Despite this treatment history, the efficacy profile of exemestane was similar to that reported by previous studies. In the multicenter, randomized phase III EFECT trial, 342 patients who received exemestane as a second-line hormone therapy after failure of NSAI treatment showed a median time to progression of 3.7 months (ORR, 6.7%; CBR, 31.5%) [6]. In a randomized phase III study of 182 patients, Paridaens et al. [4] reported a PFS of 9.9 months using exemestane as a first-line hormone therapy. Retrospective studies evaluating the efficacy of exemestane after failure of NSAI treatment reported PFS of 4 to 5 months (ORR, 3.3%-5.6%; CBR, 46.3%-46.6%) [9,10]. Our findings suggest that exemestane is similarly effective for hormone receptor-positive MBC in Korean women.
We compared survival and treatment responses of NSAI-sensitive and NSAI-resistant patients to determine whether clinical outcomes after exemestane treatment were related to the outcomes of previous NSAI treatment. We observed no difference in either CBR or median PFS between the two groups (p=0.381 for CBR, chi-square test; p=0.164 for PFS, log-rank test). Although NSAI-resistant patients showed numerically longer PFS (11.1 months vs. 5.5 months), this trend was not statistically significant and was biased by a larger proportion of censored patients in the NSAI-resistant group (6 of 27 [22.2%] vs. 14 of 85 [16.5%]); most were receiving exemestane at the data cutoff point. The multivariate analysis also showed no correlation between NSAI sensitivity and PFS (Table 4), suggesting that exemestane could be an effective treatment option for tumors resistant to NSAIs. A lack of cross-resistance between exemestane and NSAIs has been reported in several studies. In these studies, patients with tumors resistant to aminoglutethimide, anastrozole, or letrozole showed a median time to progression of 3.2 to 5.1 months and CBR of 24.3% to 54.8% [11-13]. The lack of cross-resistance may be due to the androgenic effects of exemestane, but the mechanism remains unclear [14].
In this study, CBR was the highest in patients with bone-dominant disease (62.7%), followed by patients with asymptomatic viscera-dominant disease (50.0%), lymph node or soft tissue-dominant disease (29.2%), and symptomatic viscera-dominant disease (17.6%). Symptomatic visceral metastases respond less well to hormone therapy than soft tissue or bone metastases [15,16]. Patients with visceral metastasis were analyzed separately in the EFECT study, and the results showed that their CBR following exemestane treatment (27.2%) was lower than that of patients without visceral metastasis (40.7%) [17]. In a retrospective study, Steele et al. [9] also reported a lower CBR in patients with visceral disease (33.3%) compared with patients with nonvisceral disease (56.5%). Our findings are consistent with these results. However, of note, patients with asymptomatic viscera-dominant disease showed a favorable outcome following exemestane treatment (CBR, 50.0%), suggesting that exemestane could be an affordable alternative to chemotherapy in this patient group. Lymph node- or soft tissue-dominant disease also showed a somewhat worse response to exemestane, but this difference was not significant. This finding may have been partly influenced by an imbalance in baseline characteristics, as patients with lymph node- or soft tissue-dominant disease tended to have a large tumor burden. Prospective studies are needed to better understand the influence of the dominant metastatic site on clinical outcomes after exemestane treatment.
Our study had several limitations. First, the retrospective design made it difficult to compare functional status, symptoms, and toxicity profiles of patients. Second, this was a single-center study conducted at Seoul National University Hospital, a tertiary referral hospital in Korea. Therefore, use of intensive treatment and patient compliance may have been above average. Third, we were unable to obtain information about bone health for most patients. Osteoporosis can significantly reduce quality of life in patients receiving exemestane and may be associated with treatment duration. Finally, the small sample size may have limited the ability to derive statistically significant results and correct for differences in subgroup characteristics. However, this is the first study that has provided efficacy data for exemestane use in Korean women.
In conclusion, the steroidal AI exemestane was an effective option for postmenopausal, hormone receptor-positive MBC in Korean women. Exemestane 25 mg once daily may be an attractive alternative to cytotoxic chemotherapy, even for tumors that are resistant to NSAIs.
Figures and Tables
ACKNOWLEDGEMENTS
We would like to thank all participating patients in the Seoul National University Hospital.
Notes
References
1. Rugo HS. The breast cancer continuum in hormone-receptor-positive breast cancer in postmenopausal women: evolving management options focusing on aromatase inhibitors. Ann Oncol. 2008. 19:16–27.

2. Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009. 7:122–192.

3. Bertelli G, Gangadhara S. Exemestane in postmenopausal women with early or advanced breast cancer: a review. Expert Opin Pharmacother. 2010. 11:1933–1942.

4. Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008. 26:4883–4890.

5. Kaufmann M, Bajetta E, Dirix LY, Fein LE, Jones SE, Zilembo N, et al. The Exemestane Study Group. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. J Clin Oncol. 2000. 18:1399–1411.

6. Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008. 26:1664–1670.

7. Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006. 4:Suppl 3. S1–S22.

8. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009. 101:736–750.

9. Steele N, Zekri J, Coleman R, Leonard R, Dunn K, Bowman A, et al. Exemestane in metastatic breast cancer: effective therapy after third-generation non-steroidal aromatase inhibitor failure. Breast. 2006. 15:430–436.

10. Carlini P, Michelotti A, Ferretti G, Ricci S, Giannarelli D, Pellegrini M, et al. Clinical evaluation of the use of exemestane as further hormonal therapy after nonsteroidal aromatase inhibitors in postmenopausal metastatic breast cancer patients. Cancer Invest. 2007. 25:102–105.

11. Lønning PE, Bajetta E, Murray R, Tubiana-Hulin M, Eisenberg PD, Mickiewicz E, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: a phase II trial. J Clin Oncol. 2000. 18:2234–2244.

12. Bertelli G, Garrone O, Merlano M, Occelli M, Bertolotti L, Castiglione F, et al. Sequential treatment with exemestane and non-steroidal aromatase inhibitors in advanced breast cancer. Oncology. 2005. 69:471–477.

13. Chin YS, Beresford MJ, Ravichandran D, Makris A. Exemestane after non-steroidal aromatase inhibitors for post-menopausal women with advanced breast cancer. Breast. 2007. 16:436–439.

14. Lønning PE. Lack of complete cross-resistance between different aromatase inhibitors: a real finding in search for an explanation? Eur J Cancer. 2009. 45:527–535.

15. Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000. 59:271–278.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download