Abstract
Purpose
This study compared the survival outcomes of different treatment methods for the ipsilateral breast of occult breast cancer (OBC) patients with axillary lymph node metastasis.
Methods
A retrospective study was conducted in which forty OBC patients with axillary lymph node metastasis were identified out of 15,029 patients who had been diagnosed with a primary breast cancer at between 1992 and 2010. The patients were categorized into three treatment groups based on ipsilateral breast management: breast-conserving surgery (BCS) (n=17), mastectomy (n=12), and nonsurgical intervention with or without radiation therapy (No surgery with or without radiation therapy [No Op±RT]) (n=11). All patients underwent axillary lymph node dissection. Cases were evaluated based on treatment and potential prognostic factors with respect to overall survival (OS) and disease-free survival (DFS).
Results
During the follow-up period (median follow-up of 71.5 months), the overall OS and DFS were 76.9% and 74.9%, respectively. The 5-year treatment-specific OS was 72.0% for the BCS group, 74.0% for the mastectomy group, and 87.5% for the No Op±RT group (log-rank p=0.49). The 5-year DFS was 70.6% for the BCS group, 66.7% for the mastectomy group, and 90.9% for the No Op±RT group (log-rank p=0.36). Recurrence rates for the BCS and No Op±RT groups were 5.9% and 18.2%, respectively. Histologic grade and lymph node status were inversely correlated with DFS (log-rank p=0.04 and p<0.01, respectively).
Conclusion
There was no difference in survival outcomes between the three treatment methods for the ipsilateral breast (mastectomy, BCS, and No Op±RT) of OBC patients with axillary lymph node metastasis. A large-scale multicenter study is needed to validate the results from this small retrospective study.
Occult breast cancer (OBC) is generally defined as a tumor that has been clinically diagnosed as having palpable axillary lymphadenopathy with no detection of primary tumor(s) in the breast by either palpation or mammography [1-3]. The current treatment approaches for OBC generally conform to the National Comprehensive Cancer Network (NCCN) Guidelines, and there appears to be a consensus among clinicians that axillary lymph node dissection (ALND) is appropriate for the detection of ipsilateral metastatic axillary lymph nodes. However, there have been reports that any treatment of the ipsilateral breast, including breast-conserving surgery (BCS), mastectomy, or nonsurgical intervention with or without radiation therapy (No Op±RT), can improve survival rates compared with nontreatment. Nevertheless, few studies have examined differences in survival outcomes between these treatment approaches for OBC patients [1,4-6].
An incidence rate of about 0.3% to 1% per year has been typically reported for OBC, and the introduction of more sensitive testing has increased the detection rate of these tumors [7-9]. However, the possibility that improved diagnostic rates for OBC might lead to increased survival rates has not yet been demonstrated. Furthermore, studies currently reported in the literature that examined factors relating to survival rates are inadequate. In Korea, there has been only one previous investigation of OBC, published in 1998, which assessed only seven cases [10]. A systematic analysis of the treatment and survival of a sufficiently large number of OBC patients is therefore lacking. As such, we endeavored to analyze the breast cancer database from the Department of Breast Surgery at the Asan Medical Center. To our knowledge, this is the first systematic report of OBC in Korea. Our investigation utilized comparative analyses to assess survival outcomes with respect to various approaches for treatment of the ipsilateral breast in OBC patients. We additionally evaluated several potential prognostic factors for OBC patients.
Using patient records, we identified 15,029 breast cancer patients who had been treated between 1992 and 2010 at the Breast Cancer Center at the Asan Medical Center. To be included in this retrospective cohort of OBC, patients were required to have palpable axillary lymphadenopathy with no palpable tumor in the breast, no visible lesion on a mammography, and a histopathologic finding of a manifestation of primary breast cancer from an axillary tumor biopsy. Fifty breast cancer cases met these criteria. Cases were excluded from the study if they did not undergo ALND (3 cases), were diagnosed with a stage IV tumor (2 cases), or had a prior history of diagnosis for another cancer type or history of treatment for cancer other than a primary breast cancer (5 cases). Ten of the OBC cases were excluded from the study based on these criteria, leaving a total of 40 patients available for the final analyses.
To obtain overall survival (OS) and disease-free survival (DFS) rates based on ipsilateral breast treatment modality, patients were retrospectively categorized into three groups based on the ipsilateral treatment received: BCS (n=17), mastectomy (n=11), and No Op±RT (n=12). All subjects underwent ALND, and adjuvant treatments were carried out postoperatively in accordance with general therapeutic guidelines. We performed additional survival analyses in which we subdivided OBC patients into two groups based on results from additional imaging: those for whom a breast lesion was detected on ultrasonography (USG) or magnetic resonance imaging (MRI) (n=18) and those with no detectable breast lesion on additional USG or MRI (n=22). Clinicopathologic parameters including age, histologic grade and type, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), number of positive lymph nodes, and tumor subtype were analyzed to evaluate factors associated with survival for OBC patients.
Pearson chi-square test was used to assess univariable differences in the frequency table and OBC diagnosis by performing USG. OS and DFS rates were estimated by using Kaplan-Meier survival functions, with the log-rank and chi-square tests to evaluate differences between curves. The Cox proportional hazards model was used to examine putative prognostic factors for OBC. Statistical significance was considered where p<0.05. Statistical analyses were conducted by using SAS software version 9.2 (SAS Institute Inc., Cary, USA). Our study protocols were approved by the Institutional Review Board of the Asan Medical Center (2013-0347).
The median age of our OBC patient cohort was 50 years (range, 33-64 years) and the median follow-up period after surgery was 71.5 months (range, 5-205 months). Clinicopathologic characteristics are presented according to treatment modality in Table 1. There were significant age differences in frequency of surgical approach (p=0.01). Patients who were aged 50 years or older were most frequently treated with BCS (76.5%), whereas those younger than 50 years typically underwent a mastectomy (83.3%). Frequencies for No Op±RT were similar for patients above and below 50 years of age (45.5% vs. 54.5%, respectively). There was no significant difference in pathologic T stage between the BCS group and mastectomy group (p=0.62). No significant difference was observed in the frequency of histologic grade and type, number of metastasized axillary lymph nodes, ER, PR, HER2, or tumor subtype between the three treatment groups (Table 1). There were also no significant differences in the chemotherapies or hormone therapies administered between treatment groups (Table 2).
The 5-year OS was 76.9% and the 5-year DFS was 74.9% for all OBC patients in our cohort. The 5-year OS for the BCS, mastectomy, and No Op±RT groups was 72.0%, 74.0%, and 87.5%, respectively, with no significant difference observed between them (p=0.49) (Figure 1). The 5-year DFS for BCS, mastectomy, and No Op±RT groups was 70.6%, 66.7%, and 90.9%, respectively (p=0.36) (Figure 1). The recurrence rate in the ipsilateral breast for the No Op±RT group and in the BCS group was 18.2% and 5.9%, respectively (Table 3).
All 40 OBC patients in our cohort underwent an additional USG examination to evaluate breast lesions and 22 patients received an additional MRI examination. In this study, OBC, defined as no detectable breast cancer lesion(s) on mammography, accounted for 0.27% of all breast cancer diagnoses. Following USG and MRI, previously undetected breast lesions on mammography were newly identified in 18 (45%) in our cohort. There were no significant survival differences between patients for whom the breast lesion was detected by performing additional USG or MRI and those with no detectable breast lesion (OS, p=0.69; DFS, p=0.92) (Figure 2).
To identify potential prognostic factors for OBC, we examined the association of age, tumor size, histologic grade and type, ER, PR, HER2, number of positive lymph nodes, and tumor subtypes with survival outcomes among patients in our cohort. Histologic grade and lymph node status were inversely correlated with DFS (p=0.04 and p<0.01, respectively) (Figure 3). There were no significant differences in the DFS rates in terms of age, tumor size, histologic type, ER, PR, HER2, or tumor subtypes. We were unable to assess the association of prognostic factors with OS due to the limited number of events (deaths) observed in our study.
The relatively low incidence of OBC presents a major challenge in conducting an independent study at a single institution. In principle, a multi-centered retrospective study would be desirable in this circumstance. However, in practice, institutional differences in approaches to patient management and laboratory follow-up procedures make it difficult to compile a uniform multi-center clinical database, and analysis of results from incongruent treatment modalities can be challenging. The only previous Korean study on OBC, conducted by Ahn et al. in 1998 [10], included only seven OBC patients. As such, a systematic analysis of OBC cases within the Korean population is highly desirable and thus was the aim of our present study, which represents the first systematic report of OBC in Korea. A major strength of our approach is that were able to minimize potential bias by using a database from a single center. Furthermore, we found that three different methods of treatment for the ipsilateral breast of OBC patients (mastectomy, BCS, and no surgery with/without radiation therapy) resulted in similar survival outcomes.
The classic definition of OBC is a primary breast cancer with palpable metastasis to the axillary node but no detectable breast lesion(s) detected on physical examination or mammography. Although USG and MRI are usually used to identify lesions that have been previously undetected by performing mammography as recommended in the NCCN Guidelines, they are not yet included as part of this case definition. Treatments for our OBC patient cohort were carried out in concordance with the methods specified for stage II (N1) and stage III (N2, N3) breast cancer patients in the NCCN Guidelines. It has also been reported that no statistically significant difference exists between survival rates of OBC patients and those of general breast cancer cases [4]. There seems to be a broad consensus among clinicians regarding axillary lymph node dissection, additional radiation therapy, and chemotherapy as appropriate interventions for metastatic lesions in the axillary region. However, there is currently no definitive treatment for OBC, only recommendations [6]. As is implied by the term "occult," the issue with treatment modalities for OBC is the extent of tumor resection that is reasonable given that these patients have lesions that do not appear on standard imaging tests.
The treatment modalities for the ipsilateral breast that we evaluated in our patient cohort included BCS, mastectomy, and No Op±RT [1,6]. It is generally accepted that ipsilateral breast lesions should be treated by any means possible since untreated breast lesions turn out to be a tumor in an estimated 14% to 83% of cases [2,5,11]. Furthermore, the survival rate for breast cancer patients who are observed but not treated is poorer compared to that of patients who undergo either a mastectomy or radiation therapy [5,12,13]. Based on the findings of various reports, approximately 37% (with estimates ranging from 0% to 57%) of post-ALND patients for whom no breast lesion was detected and who were observed but received no treatment eventually developed breast cancer [2,3, 14].
Although the long-standing conventional treatment for OBC has been an ipsilateral mastectomy, in recent years, conservative treatments such as BCS or radiation therapy have increasingly been applied [5,15]. Wang et al. [5] reported that the recurrence rate for breast cancer patients who had undergone a mastectomy and who had been only observed was 26% and 77%, respectively. In addition, they found that DFS differed significantly between mastectomy-treated patients and observation-only patients, with respective median survival times of 76 months and 23 months. However, Walker et al. [1] reported that the 10-year cancer-specific survival rate for OBC patients treated with mastectomy and BCS was 73.9% and 75.5%, respectively, concluding that OBC might be properly treated with either mastectomy or BCS. In contrast, Vilcoq et al. [16] and Lee et al. [17] have reported breast cancer recurrence occurred in 12% to 33% of patients who had undergone radiation therapy. Vlastos et al. [13] and Campana et al. [18] also reported no significant difference in the recurrence or survival rates between radiation therapy-treated and mastectomy-treated breast cancer patients, with both sets of authors concluding that radiation therapy could successfully replace mastectomy.
In our cohort, we observed recurrence in the ipsilateral breast among 18.2% of patients from the No Op±RT group and 5.9% from the BCS group. One of the two recurrent cases in the No Op±RT group did not receive radiation therapy to the breast. This suggests that surgery is more effective for local control of the breast cancer although our study lacks statistical power due to the relatively small sample size. Nevertheless, no definitive treatment for the ipsilateral breast in OBC patients has yet been established [1,5,19]. The OS and DFS rates that were observed in our analysis of a Korean OBC cohort suggest that the various approaches to ipsilateral breast treatment do not affect survival outcome, which is in-line with similar reports from other countries [1,5,19]. Considering the fact that most nonsurgically treated patients underwent radiation therapy, these findings may be a result of undetected breast lesions being sufficiently small that they could be treated effectively with radiation therapy in the absence of surgical intervention.
In 1907 when Halsted [20] first described OBC, the most sensitive equipment used to detect breast lesions was mammography. More than a century later, more sensitive methodologies such as USG, MRI, positron emission tomography, and breast scans are available for detection of breast lesions. If a primary breast lesion can be detected, it is logical to perform BCS, as is done for patients with general breast cancer [1,13,15].
Physiologically, breasts in Asian women are relatively more dense and smaller in size when compared with women from Western countries. These characteristics create a disadvantage in terms of more frequent false negative results on mammography among Asian women [21,22]. Hence, MRI and USG could supplement mammography for the evaluation of OBC patients in Asia, including Korea [9,23]. In our study, breast lesions previously undetected on mammography were identified on USG or MRI in 45% of OBC patients. However, there were no significant survival differences between patients for whom a breast lesion was detected by performing USG or MRI and those for whom there was no detectable breast lesion. A possible explanation for this observed lack of survival difference could be that additional breast lesions detected by performing USG and MRI are mostly quite small and thus may have less impact on patient outcome. However, the impact of other prognostic factors, such as the lymph node metastasis or histologic grade may be of greater importance; further studies are needed to better clarify this relationship.
Prognostic factors for OBC have not yet been clearly elucidated due to the small number of available patients and the lack of prospective data. Generally, the number of lymph node metastases is the most important clinical factor in outcome prediction for breast cancer patients. It has reported that breast cancer patients with metastatic involvement of less than four lymph nodes have a higher rate of survival than those with more than four nodal metastases. This is supported by our study results and the findings of others [1,5,13,15]. The study of Baron et al. [15] reported that hormone receptor-positive breast cancer cases tended toward a better prognosis than patients with hormone receptor-negative tumors, although their results were not statistically significant. The histologic grade of the breast cancer was found to be associated with the survival rate in our current study. However, it was difficult to obtain information on histologic tumor grade because many patients had lymph node metastasis only with no lesion in the breast.
Limitations of this study include the relatively low number of OBC cases in our single study investigation, despite being conducted at the largest volume hospital in Korea. However, while a multi-center study using the nationwide Korean Breast Cancer Society database would allow for larger sample-size, it can be problematic due to the heterogeneity of the data, insufficiency of available clinical information, and potential for selection bias. Our single breast cancer database may have heightened the reliability of our data, given that our records were documented systematically. Additionally, it was difficult to stipulate the concrete indications for different breast treatments in patients with OBC due to the small sample-size and limited length of follow-up in our retrospective cohort. Further studies are needed to evaluate differences in survival rates and prognostic factors, based on the clinical characteristics and surgical methods for OBC in Korea, in large, well-designed, multi-centered cohorts.
In conclusion, this study suggests that three methods of treatment for the ipsilateral breast of OBC patients with axillary lymph node metastasis (BCS, mastectomy, and No Op±RT) do not yield differential survival outcomes. These findings should be validated through additional large-scale multicenter studies.
Figures and Tables
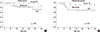 | Figure 1Overall survival (A) and disease-free survival (B) curves of different treatment methods to the ipsilateral breast in the patients with occult breast cancer. BCS=breast-conserving surgery; No Op±RT=no surgery with or without radiation therapy. |
 | Figure 2Overall survival (A) and disease-free survival (B) curves between the breast lesion detected on additional ultrasonography (USG) or magnetic resonance imaging (MRI) group and the no detectable breast lesion on additional USG or MRI group. |
 | Figure 3Disease-free survival curves according to histologic grade (A) and number of positive lymph nodes (LNs) (B). |
Table 1
Clinicopathological comparison among three treatment groups to the ipsilateral breast in the patients with occult breast cancer

BCS=breast-conserving surgery; No Op±RT=no surgery with or without radiation therapy; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; LN=lymph node; HR=hormone receptor; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma.
*All TX cases; †p-value between the mastectomy group and the BCS group; ‡One case of T2 was included.
References
1. Walker GV, Smith GL, Perkins GH, Oh JL, Woodward W, Yu TK, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010; 116:4000–4006.

2. Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992; 70:504–508.

3. van Ooijen B, Bontenbal M, Henzen-Logmans SC, Koper PC. Axillary nodal metastases from an occult primary consistent with breast carcinoma. Br J Surg. 1993; 80:1299–1300.

4. Carlson RW, Edge SB, Theriault RL. NCCN Breast Cancer Practice Guidelines Panel. NCCN: breast cancer. Cancer Control. 2001; 8:6 Suppl 2. 54–61.
5. Wang X, Zhao Y, Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010; 16:32–37.

6. Khandelwal AK, Garguilo GA. Therapeutic options for occult breast cancer: a survey of the American Society of Breast Surgeons and review of the literature. Am J Surg. 2005; 190:609–613.

7. Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005; 12:1045–1053.

8. Olson JA Jr, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000; 7:411–415.

9. Chan SW, Cheung PS, Chan S, Lau SS, Wong TT, Ma M, et al. Benefit of ultrasonography in the detection of clinically and mammographically occult breast cancer. World J Surg. 2008; 32:2593–2598.

10. Ahn SH, Park JM, Gong G. Axillary lymph node presentation with occult breast carcinoma. J Korean Surg Soc. 1998; 54:482–487.
11. Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000; 47:143–147.

12. Barton SR, Smith IE, Kirby AM, Ashley S, Walsh G, Parton M. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer. 2011; 47:2099–2106.

13. Vlastos G, Jean ME, Mirza AN, Mirza NQ, Kuerer HM, Ames FC, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001; 8:425–431.

14. Kemeny MM, Rivera DE, Terz JJ, Benfield JR. Occult primary adenocarcinoma with axillary metastases. Am J Surg. 1986; 152:43–47.

15. Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990; 125:210–214.

16. Vilcoq JR, Calle R, Ferme F, Veith F. Conservative treatment of axillary adenopathy due to probable subclinical breast cancer. Arch Surg. 1982; 117:1136–1138.

17. Lee WJ, Chu JS, Chang KJ, Chen KM. Occult breast carcinoma: use of color Doppler in localization. Breast Cancer Res Treat. 1996; 37:299–302.
18. Campana F, Fourquet A, Ashby MA, Sastre X, Jullien D, Schlienger P, et al. Presentation of axillary lymphadenopathy without detectable breast primary (T0 N1b breast cancer): experience at Institut Curie. Radiother Oncol. 1989; 15:321–325.

19. He M, Tang LC, Yu KD, Cao AY, Shen ZZ, Shao ZM, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012; 38:1022–1028.

20. Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907; 46:1–19.

21. El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol. 2001; 11:257–263.

22. del Carmen MG, Halpern EF, Kopans DB, Moy B, Moore RH, Goss PE, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007; 188:1147–1150.

23. Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010; 7:18–27.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download