Abstract
Purpose
For patients with breast carcinoma, immunohistochemical markers are important factors in determining the breast cancer subtype and for establishing a therapeutic plan, including the use of neoadjuvant chemotherapy (NACT). However, it is not clear whether the expression of certain markers changes after NACT.
Methods
We assessed estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki-67, p53, and Bcl-2 expression in specimens from 345 breast cancer cases before and after NACT. We analyzed the association between response to NACT and the expression of the markers in pre-NACT specimens. We also compared the expression between pre- and post-NACT specimens.
Results
ER and PR expression was negatively associated with pathological complete response (pCR). HER2 was associated with pCR in all cases, but the association was lost when the cases were subdivided according to hormone receptor status. The pre-NACT tumor size of cases with pCR after NACT was smaller than that of cases with residual disease. HER2-enriched and triple-negative breast cancers were more likely to achieve pCR than luminal A type cancers. PR expression and the Ki-67 index decreased after NACT. A decrease in the Ki-67 index was also demonstrated in hormone receptor positive and HER2-enriched subtypes, but no similar tendency was observed in the triple-negative subtype.
Conclusion
A patient with breast cancer scheduled for NACT should be assessed for the breast cancer subtype, as this will influence the treatment plans for the patient. The expression of PR and Ki-67 after NACT should be interpreted carefully because NACT tends to reduce the expression of these molecules.
In breast carcinoma, not only should classical pathologic markers such as stage and grade be evaluated, but also molecular markers such as hormone receptors (HRs) and human epidermal growth factor receptor 2 (HER2) to assist both the prediction of prognosis and the therapeutic plan [1]. Using gene expression profiling (also known as cDNA microarray), breast carcinoma can be classified into several subtypes, such as luminal A (LA), luminal B (LB), HER2-enriched (HE), and triple-negative (TN) types; each subtype has a unique biological behavior [2,3]. However, due to the high cost and the requirement for fresh frozen tissue, its widespread use in routine pathological procedures has thus far been limited. Instead, the association between clinicopathological markers and classification according to estrogen receptor (ER), progesterone receptor (PR), and HER2 expression, as measured by immunohistochemistry (IHC), is more widely used, as it has revealed comparable results in recent studies [4,5]. Recently, Ki-67 proliferation index has become another important marker used to distinguish more aggressive cases among HR-positive breast carcinomas [6].
Neoadjuvant chemotherapy (NACT) is used for patients with locally advanced breast carcinoma as well as for patients with metastatic or inoperable breast carcinoma to reduce tumor size and subsequently improve breast-conserving surgery rates [7]. In addition, large randomized clinical trials demonstrated that patients who display a pathological complete response (pCR) after NACT have better survival than those with residual disease (RD) [8]. It is recommended that an assay for HRs and HER2 should be performed in a resection specimen after NACT if they were not expressed in the pre-NACT biopsy specimen [9]. However, it is unclear whether IHC expression of certain markers changes after NACT.
In this study, we assessed the expression of IHC markers in pre-NACT biopsy specimens and, if possible, in a patient's post-NACT resection specimen containing RD. We evaluated 1) the association between marker expression in pre-NACT specimens and pathological responses to NACT and 2) changes in the expression of IHC markers in the pre-NACT and post-NACT specimens, in the total number of cases, and in each subtype classified according to the IHC results.
Three hundred forty-four patients including one with bilateral breast carcinoma who had received NACT for breast carcinoma at the Seoul National University Hospital, Seoul, Korea between November 2005 and April 2010 were selected retrospectively. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: H-1307-012-502). Patient age ranged from 24 to 78 years old (mean age, 46.4 years), and the diameter of the breast carcinoma before NACT ranged from 0.8 to 13.6 cm (mean, 5.14 cm). All patients had a radiologically detectable primary tumor of more than 2 cm or positive ipsilateral axillary lymph nodes. All but 11 patients had positive axillary nodes that were detected by radiologic studies. All patients underwent a core biopsy of the primary tumor prior to NACT and then received NACT comprising three to seven cycles of docetaxel (75 mg/m2) and doxorubicin (50 mg/m2); 255 patients (73.9%) received three cycles, and the remaining patients received four or more cycles. All patients underwent surgery, including mastectomy in 171 cases (49.6%) and breast-conserving quadrantectomy in 174 cases (50.4%), approximately 4 weeks after the completion of NACT. The patient with bilateral carcinoma underwent bilateral mastectomy.
All resection specimens were pathologically examined, including three-dimensional size measurements of the RD. Ipsilateral axillary lymph nodes were dissected and assessed in all but four. The average numbers of total and metastatic nodes were 14.7 (range, 1-52) and 3.82 (range, 0-37) per case, respectively. One hundred eighteen cases (34.2%) had no tumor involvement of the axillary lymph nodes. The tumor response to NACT was determined according to Sataloff criteria as follows: pCR was defined as minimal RD (<5% of tumor surface) in the primary site or no residual invasive carcinoma in the primary site and no metastasis in the axillary lymph nodes [10].
IHC was performed on both needle biopsy specimens before NACT and resection specimens after NACT. IHC studies of ER (1:100, 1D5; Dako, Glostrup, Denmark), PR (1:200, PgR636; Dako), HER2 (1:200, CB11; Leica Microsystems, Berlin, Germany), p53 (1:500, DO-7; Dako), Bcl-2 (1:200, 124; Dako), and Ki-67 (1:300, MIB-1; Dako) were performed on the 4-µm-cut sections of both specimens by using an Autostainer Link 48 (Dako). Positive staining was defined as nuclear staining in ≥1% of the tumor cells for ER and PR and in ≥50% for p53. For Bcl-2, cytoplasmic staining in ≥10% of the tumor cells was defined as positive. Only the membranous staining of tumor cells was scored for the determination of HER2 expression, and the intensity was scored as follows: 0 for negative staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining in at least 30% of the tumor cells. Fluorescence in situ hybridization (FISH) was used to assess the number of copies of HER2 for cases exhibiting 2+ or 3+ HER2 staining by performing IHC with a Vysis PathVysion kit (Abbott, North Chicago, USA). Cases with a HER2/chromosome 17 ratio ≥2.0 on performing FISH and/or HER2 expression of 3+ on performing IHC were considered to have positive HER2 expression, whereas cases which did not meet these criteria were considered HER2-negative including cases with HER2 IHC 3+ but HER2 FISH score less than 2.0. The procedures for IHC and FISH were previously described [11]. The Ki-67 proliferation index of each case was evaluated from the percentage of Ki-67-positive cells among at least 200 tumor cells.
According to the criteria suggested by Cheang et al. [6], the cases were classified into four subtypes according to the IHC results: 1) LA (ER- and/or PR-positive, HER2-negative, and Ki-67 <14%); 2) LB (ER- and/or PR-positive and either HER2-positive or Ki-67 ≥14%); 3) HE (ER- and PR-negative and HER2-positive); and 4) TN (ER-, PR-, and HER2-negative).
Statistical analysis was performed by using SPSS version 19.0 (IBM Corp., Armonk, USA). Associations between the response to chemotherapy and other variables were assessed by using the chi-square or Fisher exact tests in a 2×2 table of variables. Associations between the response to NACT and the Ki-67 index were assayed by using the independent Student t-test. Associations between IHC markers before and after NACT were evaluated by using the McNemar test for ER, PR, HER2, p53, and Bcl-2 and a paired Student t-test and Wilcoxon signed-rank test for the Ki-67 index.
In total, 46 of the 345 cases (13.3%) displayed pCRs: 24 cases had no residual invasive carcinoma or ductal carcinoma in situ (DCIS), seven cases had residual DCIS only, and 15 cases had minimal residual invasive carcinoma. The patient with carcinoma in both breasts did not achieve pCR. First, we assessed the association between the response to NACT and the expression of clinicopathological variables in the biopsy specimen before NACT. ER and PR were assessed in all cases, HER2 was assessed in 341 cases (98.8%), and p53 and Bcl-2 were assessed in 331 cases (95.9%). The response to NACT was negatively correlated with the expression of ER (p=0.0001) and PR (p=0.0184). The cases with HER2 overexpression displayed better responses to NACT (p=0.0136). Overexpression of p53, which is generally caused by a TP53 mutation, and Bcl-2 expression were not associated with the response to NACT (p=0.2372 and p=0.0834, respectively). The presence of radiologically detectable axillary nodes and the number of NACT cycles were not associated with the response to NACT (p=0.1697 and p=0.1491, respectively). The average tumor size before NACT in the cases that achieved pCRs was smaller than that of the cases with RD after NACT (p=0.0027). The average age and Ki-67 index of the tumor, the latter of which was assessed in 332 cases (96.2%), were not significantly correlated with pCR or RD (p=0.5539 and p=0.4444, respectively). Classification according to IHC was determined, and the findings were as follows: 104 (30.1%), 77 (22.3%), 55 (15.9%), and 109 (31.6%) lesions were classified as LA, LB, HE, and TN, respectively. We estimated the association between the NACT response and subtypes of breast cancer classified according to the IHC results. Tumors of the LB (9/76, p=0.0407), HE (15/56, p<0.0001), and TN types (18/109, p=0.0024) were more responsive to NACT than those of LA type (4/104) (Table 1).
Next, we analyzed the association between the response to NACT and clinicopathological variables after dividing the cases into HR-positive (LA and LB) and HR-negative (HE and TN) groups. Only smaller tumor size was associated with pCR in the HR-negative group (p=0.0016). The other variables, including HER2 overexpression (in contrast to the result for this marker in the entire cohort), were not related to pCR in either group (Table 2).
We also analyzed the association between tumor response and clinicopathological variables in the LB, HE, and TN groups. Due to the small number of cases displaying pCRs after NACT, we did not analyze the association in the LA group. In the HE group, pCR was associated with smaller tumor size before NACT (p=0.0121). In the TN group, the association between the response to NACT and pre-NACT tumor size was weaker (p=0.0442). In the LB group, however, no clinicopathological variables, including HER2 overexpression, were associated with the response to NACT (Table 3).
At least one IHC marker in the post-NACT resection specimen was assessed in 138 cases (40.0%). We evaluated and compared six IHC markers in the biopsy specimens before NACT and the resection specimens after NACT. PR expression decreased significantly after NACT (p=0.0009). In total, 19 of 45 cases (42.2%) with PR positivity in their biopsy specimens exhibited negativity after NACT (Figure 1A, B), whereas only three of 75 PR-negative cases in the biopsy specimens displayed positivity after NACT. ER expression after NACT was not changed significantly (p=0.1185). We also analyzed the changes after dividing the cases based on the subtype of their pre-NACT specimens. In the LB group, loss of ER and PR was evident after NACT (p=0.0313 and p=0.0020, respectively), whereas loss of ER and PR was less marked in the LA group (p=0.0625 and p=0.0654, respectively). In HR-negative pre-NACT specimens, only four cases gained at least one HR after NACT, including one case that gained both. There was no association of the expression of HER2, p53, and Bcl-2 with NACT, even after dividing cases by their pre-NACT subtypes (Table 4).
The average Ki-67 proliferation index decreased significantly after NACT (16.2% vs. 8.70%, p<0.0001) (Figure 1C and 1D). We also analyzed the changes according to the pre-NACT subtypes. In the LB group, a decrease in the average Ki-67 index was obvious after NACT (26.8% vs. 6.75%, p<0.0001). The average Ki-67 index also decreased after NACT in the LA (4.74% vs. 2.69%, p=0.0048) and HE groups (12.1% vs. 5.87%, p=0.0241) cases. However, in the TN group, the average Ki-67 index did not decrease significantly after NACT (23.3% vs. 18.7%, p=0.2616). These changes were similar to the results found by using the Wilcoxon signed-rank test (Table 5).
Ninety-two cases (26.7%) could be reclassified according to IHC results in the resection specimen. Twenty-eight of these 92 cases (30.4%) displayed different subtypes after NACT. Of the 22 cases that were classified as LB before NACT, nine cases were reclassified as LA after NACT. In most of these cases, the reclassification was due to a decrease in the Ki-67 index. In eight LB cases, the resection specimen after NACT had the same subtype (Table 6).
This study revealed that breast cancer subtypes are associated with the response to NACT. The overall pCR rate was 13.3%, similar to that in another study of Korean breast cancer patients [12]. The pCR rates for the HE and TN subtypes were significantly higher than for the luminal subtypes; in the luminal subtypes, the pCR rate of the LB group was higher than that of the LA group, which is consistent with other studies [13,14]. Age was not associated with pCR in the present study. Moreover, although the Ki-67 index of the LA group (all cases were <14% by definition) was lower than that of the other subtypes, it was not associated with pCR, either overall or by subtype.
HER2 overexpression according to HER2 amplification is not an independent factor for anticipating pCR after NACT. HER2 overexpression among all cases was associated with pCR in this study. When the cases were divided according to HR expression; however, HER2 did not affect the response to NACT in any group. Moreover, the response to NACT in the HER2-positive LB cases was similar to that in the HER2-negative LB cases, implying that HER2 overexpression in the LB group was not associated with pCR. The results were probably due to the differences in HER2 overexpression rates among breast cancer subtypes: the LA cases, the lowest pCR rate among subtypes, were totally negative for HER2, whereas HE cases, the highest pCR rate among subtypes, were positive for HER2. It has not been elucidated whether the HE or TN subtype is more responsive to NACT without trastuzumab, an anti-HER2 monoclonal antibody. The HE subtype was more responsive in some studies including this one, whereas the TN subtype was more responsive in others [13,14].
Since NACT was first used to treat breast cancer, a considerable number of studies have demonstrated that the expression of HRs and/or HER2 is associated with the NACT response [12-14]. However, the majority of these studies classified IHC subtypes according to HRs and HER2 only, and they did not include the Ki-67 index; HR-positive, HER2-negative cases were generally classified as LA. In addition, previous studies using gene expression profiling revealed that genes related to cell proliferation, including Ki-67 (MKI67), were overexpressed in the LB subtype, whereas HER2 was overexpressed in a small proportion of the cases of this subtype [6,15,16]. We classified cases with a higher Ki-67 index (expression by 14% or more of tumor cells) in the LB group instead of the LA group even though they were HR-positive and HER2-negative, which could explain why the pCR rates of the LA group in this study (3.85%) were lower than those in other studies [12].
We defined pCR according to Sataloff criteria, which differ from the pCR according to Chevallier criteria by also including the presence of minimal RD in the primary site. Penault-Llorca et al. [10] compared the two systems and concluded that the minimal RD after NACT did not affect the prognosis. Symmans et al. [17] assessed the RD after NACT by using the residual cancer burden index, which provided a response to NACT as a continuous parameter of the RD both in the primary site and in the lymph nodes, and they reported that the prognosis of cases with minimal RD was similar to those with no RD. All three of these criteria designate residual DCIS only after NACT as a pCR. This is supported by several studies that reported that minimal RD after NACT does not affect prognosis [18,19]. However, von Minckwitz et al. [20] recently demonstrated that cases with no residual invasive or in situ carcinoma after NACT had longer disease-free survival, but not overall survival, than those with DCIS only, which disagrees with previous studies. On the basis of their results, they suggested that pCR should be defined as no RD or no residual DCIS after NACT and that residual DCIS after NACT should not be considered to indicate a pCR. Whether residual DCIS only after NACT is indicative of a pCR requires further study.
We previously assessed IHC expression before and after NACT [11]. However, the small number of cases in that study limited the analysis, and we concluded that more cases were required for a study of this type. We could not compare cases of this study with control cases that had not undergone NACT when analyzing the association between protein expression between pre-NACT and post-NACT specimens in this study. Instead, we hypothesized that the differences in the expression of IHC markers in post-NACT resection specimens were due to tumor heterogeneity if the number of cases with expression gain (from negative to positive) were similar to those with expression loss (from positive to negative). The present study indicated that PR expression and the Ki-67 index significantly decreased after NACT. In addition, we observed that the expression of HRs was associated with a worse response to NACT. We therefore suggest that the decreased expression of PR and/or Ki-67 is associated with the loss of their expression after NACT rather than tumor heterogeneity. Moreover, we lowered the cutoff points for ER and PR expression to 1%, increasing the sensitivity, whereas most previous studies set the cutoff point at 10%. In the present study, more than 10% of tumor cells in biopsy specimens were positive for PR, but in resection specimens, the tumor cells were entirely negative in most cases with expression loss after NACT.
As reviewed by van de Ven et al. [21], many previous studies revealed that the expression of HRs changes after NACT. Some studies concluded that the expression of HR did not change, but these studies had fewer patients than those that reached the opposite conclusion. Compared with HRs, HER2 is more stable after NACT [21]. Studies by Taucher et al. [22] and Kasami et al. [23] indicated that the expression of ER and PR decreases after NACT, whereas that of HER2 does not change, which is consistent with the findings of the present study. van de Ven et al. [21] proposed several possible mechanisms for HR changes after NACT. One of the hypotheses is that a decrease in hormones such as estrogen due to NACT might cause downregulation of HRs in the tumor and subsequently lead to estrogen-independent growth because ovarian dysfunction has been the most significant problem in premenopausal breast cancer patients treated with chemotherapy [24].
However, loss of HR may be one of the mechanisms for the suppression of tumor progression by NACT rather than resistance to NACT. In the present study, the Ki-67 index, as well as PR expression in RD, decreased after NACT, which was consistent with the results of other studies [22,25,26]. In addition, the present study revealed for the first time that changes in the Ki-67 index in RD varied according to breast cancer subtypes after NACT. LA and LB cases exhibited decreased expression of Ki-67 in RD, whereas TN cases failed to display a similar decrease. These results suggest that NACT can control the proliferation of RD cells of HR-positive subtypes, although NACT cannot kill the cancer cells, whereas RD cells in TN cases after NACT are either intrinsically resistant to the chemotherapeutic agents or acquire resistance, which may be the main reason for treatment failure. It has been reported that a low post-NACT Ki-67 index or a significant decrease in the Ki-67 index after NACT is associated with better prognosis in breast cancer [25,26]. In addition, it has been reported that breast cancers of the HE and TN subtypes have a worse prognosis than those of the LA and LB subtypes although the clinical and/or pathologic responses of the former subtypes after NACT are better [27].
Thus far, little is known about why the expression of HR changes after NACT. Miyoshi et al. [28] reported that intratumoral aromatase and one of its inducers, tumor necrosis factor-α, are significantly downregulated in patients with breast cancer who display a response after docetaxel treatment. They suggest that docetaxel-induced antitumor activity is mediated through the downregulation of intratumoral aromatase expression, which results in the suppression of intratumoral PR expression. Further research on the prognosis of patients with breast cancer who exhibited a decrease in PR expression and the Ki-67 index after NACT is required.
The classification of breast cancer subtypes by performing IHC of the resection specimen after NACT should be interpreted with caution. For example, only eight cases that were classified as LB on the basis of the biopsy specimen were classified as the same subtype when the resection specimen was examined. Nine LB cases were reclassified as LA in the resection specimen after NACT, mainly due to a decrease in the Ki-67 index induced by NACT. However, we consider that it is also useful to assess the expression of markers, including ER, PR, and HER2, in the resection specimen to aid decisions concerning the treatment plan after surgery. If the expression of HRs in the RD cells changes from positive to negative, hormone therapy after surgery may be less effective. Hirata et al. [29] recently reported that after adjuvant hormone therapy, the survival of patients whose HR status had changed (either positively or negatively) in the resection specimen after NACT did not differ from that of patients whose HR status remained positive after NACT. By contrast, Chen et al. [30] reported that following adjuvant hormone therapy, the survival of patients whose HR status changed after NACT was poorer than that of patients whose HR status remained positive after NACT.
In summary, a patient with breast cancer who is scheduled for NACT should be assessed for the subtype of breast cancer before NACT, by using either cDNA microarray or IHC, for planning treatment. Patients with LA subtype breast cancer have a poor response to NACT, and they should instead undergo surgery or neoadjuvant hormone therapy, whereas NACT will be helpful for patients with HE or TN subtype breast cancer. Assessment of the Ki-67 index in the biopsy specimen before NACT is necessary in HR-positive breast cancer because the LB subtype is more responsive to NACT than the LA subtype irrespective of the HER2 expression status. In addition, PR expression and the Ki-67 index in the resection specimen after NACT should be interpreted carefully because NACT tends to cause a decrease in the expression of these molecules.
Figures and Tables
Figure 1
Immunohistochemical stains for progesterone receptor (PR) (A, B) and Ki-67 (C, D) before (A, C) and after (B, D) neoadjuvant chemotherapy (NACT) (×200). PR is positive in the biopsy specimen before NACT (A) and negative in the resection specimen after NACT (B). The Ki-67 index of the tumor is about 70% in the biopsy specimen before NACT (C), while it is significantly decreased in the resection specimen after NACT (D).
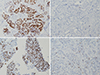
Table 1
Association between response to neoadjuvant chemotherapy and clinicopathological markers before neoadjuvant chemotherapy
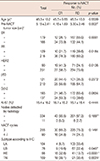
NACT=neoadjuvant chemotherapy; CR=complete response; RD=residual disease; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; IHC=immunohistochemistry; LA=luminal A; LB=luminal B; HE=HER2-enriched; TN=triple-negative.
*Mean±SD; †Fisher exact test; ‡Compared with luminal A type.
Table 2
Association between response to neoadjuvant chemotherapy and clinicopathological markers before neoadjuvant chemotherapy in hormone receptor-positive and hormone receptor-negative groups

Table 3
Association between response to neoadjuvant chemotherapy and clinicopathological markers before neoadjuvant chemotherapy in each subtype

Table 4
Changes of immunohistochemistry expression in the residual carcinoma after neoadjuvant chemotherapy

References
1. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007; 25:5287–5312.

2. van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002; 347:1999–2009.

3. Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010; 123:725–731.

4. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010; 7:e1000279.

5. Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012; 15:203–210.

6. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750.

7. Liu SV, Melstrom L, Yao K, Russell CA, Sener SF. Neoadjuvant therapy for breast cancer. J Surg Oncol. 2010; 101:283–291.

8. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.

9. Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012; 19:1508–1516.

10. Penault-Llorca F, Abrial C, Raoelfils I, Cayre A, Mouret-Reynier MA, Leheurteur M, et al. Comparison of the prognostic significance of Chevallier and Sataloff's pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum Pathol. 2008; 39:1221–1228.

11. Lee HC, Lee JO, Park IA. Changes in protein expression in breast cancer after anthracycline-based chemotherapy. Korean J Pathol. 2007; 41:165–170.
12. Kim SI, Sohn J, Koo JS, Park SH, Park HS, Park BW. Molecular subtypes and tumor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Oncology. 2010; 79:324–330.

13. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012; 48:3342–3354.

14. Sánchez-Muñoz A, Plata-Fernández YM, Fernández M, Jaén-Morago A, Fernández-Navarro M, de la Torre-Cabrera C, et al. The role of immunohistochemistry in breast cancer patients treated with neoadjuvant chemotherapy: an old tool with an enduring prognostic value. Clin Breast Cancer. 2013; 13:146–152.

15. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003; 100:8418–8423.

16. Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006; 24:1656–1664.

17. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–4422.

18. Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006; 94:358–362.

19. Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007; 25:2650–2655.

20. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.

21. van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011; 37:422–430.

22. Taucher S, Rudas M, Gnant M, Thomanek K, Dubsky P, Roka S, et al. Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocr Relat Cancer. 2003; 10:91–98.

23. Kasami M, Uematsu T, Honda M, Yabuzaki T, Sanuki J, Uchida Y, et al. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast. 2008; 17:523–527.

24. Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012; 26:379–390.

25. Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005; 92:147–155.

26. Tanei T, Shimomura A, Shimazu K, Nakayama T, Kim SJ, Iwamoto T, et al. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2011; 37:155–161.

27. Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006; 24:1037–1044.

28. Miyoshi Y, Kim SJ, Akazawa K, Kamigaki S, Ueda S, Yanagisawa T, et al. Down-regulation of intratumoral aromatase messenger RNA levels by docetaxel in human breast cancers. Clin Cancer Res. 2004; 10:8163–8169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download