Abstract
Rhabdomyosarcoma (RMS) of the breast is rare and there is scant information about the clinical behavior and treatment strategies. We report an adolescent female patient with metastatic RMS of the breast from the anus. An 18-year-old female patient was referred to our clinic due to palpable mass in the left breast. At age seven, she was diagnosed with acute lymphoblastic leukemia and treated with chemoradiation therapy. After 10 years of complete remission state, she presented with anal mass which was diagnosed as RMS and she received chemoradiation therapy. After 1 year of complete remission state, she noticed a palpable mass in her left breast. The breast mass was diagnosed as metastatic RMS based on core needle biopsy specimen. The RMS in breast was excised for the decreasing tumor burden despite of another metastatic lesion. Although rarely reported, metastasis of RMS should be considered as a cause of breast mass. Tissue biopsy is recommended when clinically suspected lesion is detected.
Rhabdomyosarcoma (RMS) is a common soft tissue malignancies in pediatric age that accounts for 12% of all solid malignancies [1]. Whereas, RMS of breast is very rare, accounting for much less than 1% of all breast malignancies and being mainly confined to adolescent women [2]. Breast RMS is rarely primary and most often metastatic from another site. The extrammamary primary origin is usually trunk, neck, orbit, and the extremities [3]. Unfortunately, the prognosis of metastatic breast RMS is very poor and there is no consensus about the treatment of breast metastasis due to its rarity [3,4]. Therefore, to add further evaluation on this disease, we report our experience about metastatic RMS from the anus.
An 18-year-old female patient was referred to our department for evaluation of a lump in the left breast noticed 1 week earlier. The patient had a past medical history of acute lymphoblastic leukemia (ALL) and anal RMS. Eleven years earlier, at age seven, she was referred to our hospital because of fever and both knee pain. On femur and leg simple X-ray, there was irregular marginated osteolytic lesion with cortical destruction in distal femur and proximal tibia. The complete blood count was as follow: hemoglobin 12 g/dL, white blood cell count 5,000/µL with 37% of blast cell and platelet count 106,000/µL. We performed bone marrow biopsy and aspiration on iliac crest and the bone marrow specimen was packed with leukemic blasts (more than 95%). The immunohistochemical stain revealed the blast cells were positive for myeloid lineage (CD33 and CD13) and B-cell lineage (CD79a, CD20, and CD19). Finally, she had been diagnosed as biphenotype ALL and treated with the Children's Cancer Group (CCG)-106B protocol and prophylactic 18 Gy cranial irradiation [5]. During follow-up, she had remained in complete remission state. One year earlier, she visited our hospital due to an anal mass and accompanying pain. Pelvic computed tomography (CT) scan revealed an 8.5 cm sized heterogenous enhancing mass around the anus. Multiple enlarged lymph nodes were found on both inguinal area and bilateral iliac chain (Figure 1). There was no evidence of distant metastasis on fluorodeoxyglucose-positron emission tomography and computed tomography (FDG-PET/CT). For the tissue confirmation, we performed needle biopsy on the anal mass. The cells were small and round shaped on hematoxylin and eosin (H&E) stain and the mass was negative for CD3, CD20, CD79a and ETS-1, and positive for CD56, CD99 and desmin based on immunohistochemical stain. The mass was subsequently diagnosed as RMS. The patient underwent vincristine, adriamycin, and cyclophosphamide based chemotherapy. And a total of 4,500 cGy in 25 fractions of radiotherapy dose was given to the pelvis. Following treatment, FDG-PET/CT showed no uptake at the primary tumor site with complete remission state.
Upon clinical examination, a firm, round and nontender mass was found in her left breast, measuring 3×3 cm. There was no palpable mass in the contralateral breast and both axillary areas. Breast ultrasonography showed a 2.3 cm sized circumscribed oval hyperechoic mass with central hypoechoic portion (Figure 2A). Core needle biopsy was consistent with RMS. Chest, abdominal, and pelvic CT scans and FDG-PET/CT were evaluated for metastatic disease. Intense FDG uptake was present in the left breast and retrocrural space at the level of L1 verterbra (Figure 2B and 2C). For the decreasing tumor burden, mass excision was performed. The mass was well-circumscribed and, on section, shows a pale-tan solid fleshy cut surface with focal hemorrhage. No necrosis was identified in the specimen (Figure 3A). The mass was composed predominantly of primitive small round blue cells which were surrounded by collagenous fibrous septa. Some cells with rhabdoid feature were also seen (H&E stain) (Figure 3B). Immunohistochemical stain demonstrated strong positivity for CD56, CD59 and desmin as identical to anal mass (Figure 3C). The breast mass was finally diagnosed as metastatic alveolar RMS based on H&E and immunohistochemical stain. The pediatric oncologist started topotecan and cyclophosphamide chemotherapy and radiation therapy.
Breast involvement of RMS is rarely reported. The largest series about breast RMS was included 7 cases of primary breast RMS and 19 more cases with breast metastasis registered from 1972 to 1992 [6]. And the second largest series were 7 patients with metastatic breast RMS reported by Howarth et al. [7] and D'Angelo et al. [3]. Hays et al. [6] and Li et al. [8] reviewed published articles about breast RMS and most of published reports were single case reports. Therefore, the characteristics and the treatment strategies of breast metastasis have been under-ascertained. We report this rare disease with hopes that our experience will aid reader in clinical decision-making. To the best of our knowledge, this is the first report of alveolar RMS of the breast in Korean patient.
Adolescent age may delay the exact diagnosis. Hays et al. [6] reported that most of primary RMSs were initially regarded as fibroadenomas and some patients with metastatic RMS were not recognized. The gross features are round or oval and circumscribed. Because of predominant fibroglandular composition of adolescent breast, ultrasonography is reliable imaging method. However, a report described a metastatic RMS mimicking normal breast parenchyme on ultrasonography [9]. Therefore, careful physical examination, tissue biopsy and further imaging study such as magnetic resonance imaging or FDG-PET/CT are needed for accurate diagnosis.
For the differential diagnosis including other malignancies, immunohistochemical study is essential. At first, our patient was tentatively diagnosed as lymphoblastic lymphoma based on H&E stain morphology from core needle biopsy of anal mass. Immunohistochemistry was negative for CD3, CD20, CD79a, and ETS-1, and positive for CD56, CD99, and desmin. Besides RMS, although rarely reported, metaplastic carcinoma or malignant phyllodes tumor might have sarcomatous component. Unlike rhabdomyosarcoma which is aroused from stromal cell, metaplastic carcinoma is characterized by differentiation of the neoplastic epithelium into squamous cells and/or mesenchymal-looking elements. And malignant phyllodes tumor is fibroepithelial origin. Therefore, they should contain epithelial component [10]. However, in this case, the specimen from anus and breast were entirely composed of sarcomatous element.
There is no consensus about the treatment of breast RMS. Systemic chemotherapy and/or local therapy have been applied in previous reports, however it is not known if aggressive local treatment could improve prognosis. In this case, we decided to do a lumpectomy and radiation therapy despite of multiple organ involvement because we hoped that local therapy could decrease tumor burden in adjuvant chemotherapy.
Previous studies have reported that cumulative incidences of second malignant neoplasm (SMN) after treatment of childhood ALL has varied from less than 1% to 10% or more according to treatment method, duration and completeness of follow-up. Soft tissue sarcoma has been reported about 5% of total SMN [11]. Although, this single rare case could not suggest that treatment of ALL would be associated with breast rhabdomyosarcoma, clinicians should carefully checkup the patient's condition with the suspicion of SMN.
In conclusion, breast RMS is an extremely uncommon and aggressive disease. It is necessary to be aware of the risk of breast metastasis of primary RMS. Tissue biopsy is recommended when clinically suspected lesion is detected in patients with primary RMS. Immunohistochemistry is an effective method for acute diagnosis. Due to its rarity, more research is required to improve the outcome of this disease.
Figures and Tables
Figure 1
Computed tomography image of anal rhabdomyosarcoma. (A) Heterogenous enhancing mass, 8.5 cm sized in largest diameter, was noted around anus on computed tomography. (B) About 2.5 cm sized, enlarged lymph nodes were located around right iliac vessel (white arrow) and both inguinal area suggesting multiple lymph nodes metastasis.
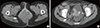
Figure 2
Image of metastatic rhabdomyosarcoma. (A) A 2.3 cm sized round and circumscribed mass was found on breast ultrasonography. Positron emission tomography/computed tomography scan demonstrates hot uptake on left upper outer breast (B) and right retrocrural area (C).
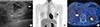
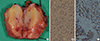
Figure 3
Histologic findings of metastatic rhabdomyosarcoma in breast. (A) The rhabdomyosarcoma was oval and well-circumscribed, 2.2 cm sized in diameter. The cut surface showed focal hemorrhage with white central compartment encircled by yellow area. The small round cells are arranged in variably sized nest by fibrous tissue septa (H&E stain, ×200) (B) and strongly stained with desmin immunohistochemical stain (desmin stain, ×200) (C).
References
1. Binokay F, Soyupak SK, Inal M, Celiktas M, Akgül E, Aksungur E. Primary and metastatic rhabdomyosarcoma in the breast: report of two pediatric cases. Eur J Radiol. 2003; 48:282–284.

2. Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma: a clinicopathologic review of 25 cases. Cancer. 1990; 66:941–944.

3. D'Angelo P, Carli M, Ferrari A, Manzitti C, Mura R, Miglionico L, et al. Breast metastases in children and adolescents with rhabdomyosarcoma: experience of the Italian Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2010; 55:1306–1309.
4. Italiano A, Largillier R, Peyrottes I, Hannoun-Levy JM, Lallement M, Thyss A. Primary embryonal rhabdomyosarcoma of the breast in an adult female. Breast J. 2005; 11:214.

5. Gaynon PS, Steinherz PG, Bleyer WA, Ablin AR, Albo VC, Finklestein JZ, et al. Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Childrens Cancer Group Study CCG-106. J Clin Oncol. 1993; 11:2234–2242.

6. Hays DM, Donaldson SS, Shimada H, Crist WM, Newton WA Jr, Andrassy RJ, et al. Primary and metastatic rhabdomyosarcoma in the breast: neoplasms of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997; 29:181–189.

7. Howarth CB, Caces JN, Pratt CB. Breast metastases in children with rhabdomyosarcoma. Cancer. 1980; 46:2520–2524.

8. Li DL, Zhou RJ, Yang WT, Wang J, Yao XH, Cheng YF, et al. Rhabdomyosarcoma of the breast: a clinicopathologic study and review of the literature. Chin Med J. 2012; 125:2618–2622.
9. Ahn SJ, Kim SK, Kim EK. Metastatic breast cancer from rhabdomyosarcoma mimicking normal breast parenchyma on sonography. J Ultrasound Med. 2010; 29:489–492.

10. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: International Agency for Research on Cancer;2012. p. 48–146.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download