Abstract
We had previously reported a close association between pathological response and the maximum tumor standardized uptake value (SUVmax) measured by 18F-fluorodeoxyglucose positron emission tomography prior to chemotherapy in estrogen receptor (ER)-positive breast cancer. We hypothesized that glucose hypermetabolism by luminal B tumors may result in chemotherapy responsiveness. Using a single-gene expression assay, TargetPrint® (Agendia) and a 70-gene expression classifier, MammaPrint® (Agendia), we divided 20 patients with ER-positive primary breast cancer into luminal A and luminal B subtypes and compared the tumor SUVmax value between the two groups. A significantly higher SUVmax was measured for luminal B tumors (n=10; mean±SD, 7.6±5.6) than for luminal A tumors (n=10; mean±SD, 2.6±1.2; p=0.01). Glucose hypermetabolism could help predict intrinsic subtyping and chemotherapy responsiveness as a supplement to ER, progesterone receptor, HER2, and Ki-67 histochemical scores.
We had previously reported the usefulness of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) for predicting the pathological complete response (pCR) of primary breast cancer in the neoadjuvant chemotherapy setting [1]. In this article, we present a close association between pathological response and the maximum tumor standardized uptake value (SUVmax) measured by FDG-PET scan prior to chemotherapy. Estrogen receptor (ER)-positive breast cancer (as determined by immunohistochemistry) presented a significantly lower baseline SUVmax (n=70; mean±SD, 6.51±3.51) than HER2 and triple-negative ones (n=24; mean±SD, 8.58±4.1; p=0.02 and n=17; mean±SD, 9.37±5.78; p=0.01, respectively). The baseline SUVmax of ER-positive tumors in which pCR was achieved (n=7; mean±SD, 9.8±4.0) was significantly higher than that in tumors in which no pCR was achieved (n=63; mean±SD, 6.4±3.1; p=0.006). Therefore, we hypothesized glucose hypermetabolism in luminal B tumors may result in chemotherapy responsiveness.
Using a single-gene expression assay, TargetPrint® (Agendia, Amsterdam, The Netherlands) and a 70-gene expression classifier, MammaPrint® (Agendia) [2,3], we divided 20 patients with ER-positive primary breast cancer into luminal A and luminal B subtypes and compared the tumor SUVmax value between the two groups. The demographics of these groups are shown in Table 1.
A significantly higher SUVmax was measured for luminal B tumors (n=10; mean±SD, 7.6±5.6) than for luminal A tumors (n=10; mean±SD, 2.6±1.2; p=0.01) (Figure 1). At the threshold of 5.0, the sensitivity and specificity of FDG-PET to identify tumors of the luminal B subtype were 60% and 100%, respectively. The area under the curve (AUC) analysis showed that SUVmax was an acceptable discriminator (AUC=0.878; 95% confidence interval [CI], 0.647-0.981) with results comparable with those of the Ki-67 labeling index (LI) (AUC, 0.878; 95% CI, 0.647-0.981), a proliferative marker used to discriminate luminal B tumors in clinical practice (Figure 2). When SUVmax and Ki-67 LI were combined, the diagnostic performance improved (AUC=0.933; 95% CI, 0.72-0.997).
Jin et al. [4] reported that among 273 breast cancer patients who received neoadjuvant chemotherapy, higher baseline SUVmax of the tumor and ER negativity were independent indicators of pCR. Despite the low number of ER-positive breast cancer patients who achieved pCR in that study, higher SUVmax in pCR than in non-pCR was in agreement with the results of our study. The role of glucose metabolism in ER-positive breast cancer was examined by Osborne et al. [5] from the Memorial Sloan-Kettering Cancer Center, who identified 43.7% of FDG SUV-correlated genes as ER signal-related genes by cDNA microarray analysis.
The present study has some limitations: the sample size was too small, including patients with relatively small tumors, which may have been a cofounding factor that affected the SUVmax. Standardization is required for the use of quantitative FDG-PET as an imaging biomarker.
Currently, FDG-PET scanning is used for noninvasive detection of metastasis. In combination with one-stop examination, which evaluates the clinical staging of primary breast cancer, FDG-PET may provide invaluable information on intrinsic subtyping and chemotherapy responsiveness in addition to that obtained from ER, progesterone receptor, HER2, and Ki-67 histochemical scores.
Figures and Tables
 | Figure 1Distribution of the maximum tumor standardized uptake value (SUVmax) of luminal A and luminal B. A significantly higher SUVmax was measured for luminal B tumors (n=10; mean±SD, 7.6±5.6) than for luminal A tumors (n=10; mean±SD, 2.6±1.2; p=0.01).
FDG=18F-fluorodeoxyglucose.
|
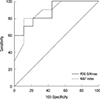 | Figure 2Diagnostic performance of predicting luminal B. The area under the curve (AUC) analysis showed that the maximum tumor standardized uptake value (SUVmax) was an acceptable discriminator (AUC=0.878; 95% confidence interval [CI], 0.647-0.981) with results comparable with those of the Ki-67 labeling index (AUC=0.878; 95% CI, 0.647-0.981).
FDG=18F-fluorodeoxyglucose.
|
Table 1
Characteristics of breast cancer patients stratified by luminal subtypes

Data are presented as mean±SD or number (%). All patients received 4 triweekly cycles of epirubicin (90 mg/m2)+cyclophosphamide (600 mg/m2) followed by 8 to 12 weekly cycles of paclitaxcel (80 mg/m2) or 4 triweekly cycles of docetaxcel (70 mg/m2).
NS=no statistic difference; ND=not described; SUVmax=the maximum value of tumor standardized uptake value.
References
1. Ueda S, Saeki T, Shigekawa T, Omata J, Moriya T, Yamamoto J, et al. 18F-fluorodeoxyglucose positron emission tomography optimizes neoadjuvant chemotherapy for primary breast cancer to achieve pathological complete response. Int J Clin Oncol. 2012; 17:276–282.

2. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.

3. Nguyen B, Cusumano PG, Deck K, Kerlin D, Garcia AA, Barone JL, et al. Comparison of molecular subtyping with BluePrint, MammaPrint, and TargetPrint to local clinical subtyping in breast cancer patients. Ann Surg Oncol. 2012; 19:3257–3263.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download