Abstract
Purpose
The aim of this study is to evaluate the clinical utility of automated breast volume scanner (ABVS) for detecting and diagnosing the breast lesions.
Methods
From December 2010 to January 2012, bilateral whole breast examinations were performed with ABVS for 139 women. Based on the Breast Imaging Reporting and Data System (BI-RADS) categories, the breast lesions were evaluated on coronal multiplanar reconstruction images using the ABVS workstation. Then, the imaging results were compared with those on conventional handheld ultrasound (HHUS) images. Histological diagnoses were performed on BI-RADS category 4 and 5 lesions.
Results
A total of 453 lesions were detected by ABVS. On the HHUS, 33 new lesions were detected but 69 lesions were not detected. BI-RADS category 2 and 3 matched to those on ABVS at 73.5% (61/83) and 85.4% (276/323). In 47 lesions of BI-RADS category 4 or 5, there was an exact match to those on ABVS. In addition, 47 lesions were classified as BI-RADS category 4 and 5, for which an ultrasound-guided core needle biopsy was performed. The malignant lesions of BI-RADS category 4 and 5 showed the following: 2/27 (7.4%) in 4A, 4/5 (80%) in 4B, 2/2 (100%) in 4C, and 13/13 (100%) in 5. The ABVS showed 21 true positives and a positive predictive value of 44.7% (21/47).
Conclusion
There was considerable agreement in the assessment of the breast lesions by ABVS and HHUS. The ABVS had advantages of high diagnostic accuracy, examiner-independence, multislice visualization of the whole breast and less time-consuming. Our results indicate that ABVS might be a useful modality in diagnosing breast lesions.
According to imaging studies for patients with breast cancer, mammography (MMG) and breast ultrasound (US) are routinely used. And breast magnetic resonance imaging is additionally used to determine the breast-conserving surgery and the TNM stage preoperatively. Of these, the MMG is accepted as the only means of reducing breast cancer mortality [1]. However, the low sensitivity of MMG for dense breasts, women younger than 50 years of age and small-sized cancers remains a major limitation [2]. The breast US is advantageous because the examiners are able to observe the mass according to desired locations, differentiate the shape and consistency of the mass, and adjust the interference signal based on their technical expertise. It is also beneficial as the breast and axilla can be examined in a one-stop process. Meanwhile, its usefulness and accuracy have been confirmed and it has been established as an essential diagnostic modality for breast cancers [3]. It is highly dependent on the examiners and there might be variations in accuracy due to the examiners. In addition, it is disadvantageous in that it is somewhat time-consuming and it has a poor reproducibility [4].
To overcome these disadvantages of breast US, state-of-the-art equipments such as an automated three-dimensional (3D) breast US have been recently introduced. An automated breast volume scanner (ABVS) (ACUSON S2000™; Siemens, Berlin, Germany) belongs to one of these equipments. This system acquires a whole series of consecutive B-mode pictures and reconstructs 3D data sets of the entire breast volume. These data can be sent to a separate workstation to be analyzed by a radiologist. It can be operated by a medical technician or radiographer [5]. The feasibility and image quality of the ABVS are good and it has been proposed as suitable for screening for breast cancers [6-8].
However, the diagnostic value of this new technique was not fully discussed in previous reports. Thus, we conducted this study to evaluate the clinical utility of the ABVS in the detection and diagnosis of breast lesions in comparison to a handheld US (HHUS) for an actual clinical setting.
During the period ranging from December 2010 to January 2012, bilateral breast examinations using ABVS and HHUS were performed in 139 women. Patients who were referred to our hospital due to specific diagnostic queries such as breast pains, palpable breast masses, and suspicious breast lesions were screened with MMG or US. We excluded the patients who were already diagnosed with breast cancer and had a history of breast surgery or comorbid disorder such as skin disorder.
In this study, we used ACUSON S2000™ ABVS in combination with 14L5BV transducer (14 MHz, 15.4 cm) which was specially designed for the equipment (Figure 1). The transducer has 768 piezoelectric crystals, and is characterized by an ability to scan automatically a 6-cm-deep and 16.8-cm-area range and to generate 318 high-resolution images up to 0.5 mm of thickness. In addition, it also has various features and functions including tissue harmonic imaging for image optimization, Advanced SieClear™ (Siemens) spatial compounding, dynamic tissue contrast enhancement, inferior-nipple image correction, and 3D image brightness auto-correction. Together with these features and functions, the images are reconstructed so that the entire breast can be visualized from different angles, and provided for secondary real-time scanning. With patients in a supine position, a wedge was used to flatten the breast, adjusted to the expected breast size (2.5 cm for A, 4 cm for B, 4.5 cm for D, 5.5 cm for D+). The scanning time was one minute per view and minor pressure was added to breast to prevent breast movement. Three standard views (coronal, longitudinal, and transverse views) were taken for each to ensure that the entire breast was covered. For the antero-posterior (AP) position, the arrow of the probe was placed centering the nipple and fixed before scanning. For the lateral position, the pod was angled with thumbs to push breast tissues from axilla toward sternum and fixed before scanning. For the medial position, the pod was angled with thumbs to push breast from sternum toward axilla and fixed before scanning. Obtained volume data were migrated from ACUSON S2000™ ABVS to the workstations and then displayed in multiplannar reconstruction (Figure 2).
In all the patients, HHUS was performed after the ABVS examination. HHUS was performed using an ACUSON Sequoia 512 (Siemens Medical Solutions, Mountain View, USA) with 17L5HD linear transducer at 15 MHz grayscale central frequency. The patients were in the supine position with the ipsilateral hand raised above the head. All examinations were performed with the US probe oriented perpendicular to the chest wall. All images were digitally recorded. For both imaging techniques, the radiologist described the shape, orientation, margin, echogenicity, echotexture, posterior acoustic transmission, boundary echo, and presence of calcifications within the mass. The radiologist then provided an American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) final assessment graded from category 1 (negative) to category 5 (highly suggestive of malignancy) [9]. We have not categorized any lesions into BI-RADS 0 since US examination completes the evaluation of all lesions. For diagnostic population, the BI-RADS category 1 to 3 were combined and rated as benign, and category 4 to 5 were rated as malignant. This was followed by the comparison of coronal multiplannar reconstruction images and HHUS using an ABVS workstation. The findings of the axillary examination were excluded. And the consistency of the mass was compared. If they were classified into the BI-RADS category 4A or higher on the ABVS or HHUS, a histopathologic examination was established by the US-guided core needle biopsy. The interpretation of images was done by experienced radiologists. This study was approved by the Institutional Review Board of our institution (IRB approval No. 2012-10-026).
The results obtained from the ABVS were compared with the results from the HHUS. The data were analyzed using descriptive statistics. The concordance rates and positive predictive values were calculated for ABVS. The software package SPSS statistics version 17.0 (SPSS Inc., Chicago, USA) was used for the statistical analysis.
A total of 453 lesions were detected on ABVS (BI-RADS category 3 or lower, 406; 4A or higher, 47). In addition, a total of 417 lesions were detected on HHUS (BI-RADS category 3 or lower, 370; 4A or higher, 47) (Table 1). Following a comparison of the two results, 33 new lesions were detected from 24 patients and 69 lesions were not detected from 25 patients on HHUS, all of which were the lesions of the BI-RADS category 3 or lower (Figure 3). We examined the concordance rate between the lesions based on the BI-RADS category. As compared with the HHUS, the lesions of BI-RADS category 2 and 3 had match to those seen on ABVS for 73.5% (61/83) and 85.4% (276/323). In the 47 lesions of BI-RADS category 4 or 5, there was an exact match to those seen on ABVS. All matched lesions showed consistent radiologic findings. In all BI-RADS category 4 or 5 lesions, a diagnosis was made on histopathologic examination. Histopathological diagnosis comprised 18 invasive ductal carcinomas, 3 ductal carcinomas in situ, 17 fibrocystic changes, 5 fibroadenomas, 2 intraductal papillomas, 1 hamartoma, and 1 chronic granulomatous mastitis.
Based on each category, the proportion of malignancy was 7.4% (2/27) in the lesions of BI-RADS category 4A, 80% (4/5) in the lesions of BIRAD category 4B, 100% (2/2) in the lesions of BI-RADS category 4C, and 100% (13/13) in the lesions of BI-RADS category 5 (Table 2). The ABVS showed 21 true positives, 26 false positives and a positive predictive value of 44.7% (21/47). No additional malignant lesions were identified in BI-RADS category 2 and 3 during 14 to 27 months follow-up on HHUS.
As a screening test for breast cancer, MMG has been universally performed. Breast US has an essential and specific role as a complementary method to MMG, and also adding diagnostic accuracy. Since the US was first used for medical purposes in 1952, it has undergone technical advancements and has been used in many specific areas [3]. With the recent advancements and universalized uses of the US, its scope of application and frequency has been increased. Breast US is now established as a standard diagnostic modality for women with clinically or radiologically detected suspicious breast lesions [10]. HHUS represents the gold standard for this examination. It has been reported, however, that HHUS are disadvantageous as it is greatly dependent on the examiner, as well as being time-consuming, and has a poor reproducibility [7].
The concept of automated breast US dates back to the 1970s when the first applicable systems were reported by Maturo et al. [8]. The ABVS is useful in obtaining 3D high-resolution images automatically for the overall breast within a shorter examination time of about 10 minutes. And as the images can be confirmed with the use of digital imaging and communications in medicine (DICOM) data, demerits of HHUS have been overcome. However, ABVS is far from representing an accepted medical practice and its accuracy and usefulness cannot be concluded [11]. Given this background, we presented our initial experiences with the latest generation of an automated breast US system, ACUSON S2000™ ABVS and compared the images between HHUS and ABVS. Thus, we estimated a diagnostic accuracy of ABVS as compared with HHUS.
Our results showed that concordance rate of 66.2% between ABVS and HHUS images in the lesions of BI-RADS category 2 or 3 and 100% in the lesions of BI-RADS category 4 or 5. Based on category 4 and 5, The ABVS had a positive predictive value of 44.7% and these results were identical to HHUS. Positive predictive value of the lesions of BI-RADS category 4C and 5 are 100%, respectively. The results of our study are similar to the results reported from other groups. Wang et al. [12] reported that the diagnostic accuracy of ABVS and HHUS in differentiating benign from malignant breast lesions is almost identical. In a study of 81 patients by Lin et al. [13], the ABVS and HHUS exhibited high sensitivity (both 100%) and high specificity (95.0% and 85.0%, respectively). In addition, Kim et al. [14] reported that there was substantial interobserver agreement in the final assessment of solid breast masses by ABVS and HHUS. And a majority of other studies have reported that the detection rate and diagnostic accuracy of ABVS were similar or higher as compared with HHUS [15-18]. With regard to the malignancy based on the BI-RADS categories that is solely dependent on ABVS, Tozaki and Fukuma [19] showed the similar results to HHUS. Our results also indicated that there was a 100% concordance rate in the lesions of the BI-RADS category 4 or higher and this led to the speculation that the ABVS has a diagnostic value. The images were actually compared between the two modalities for detecting the benign and malignant lesions, in which the results are depicted in Figure 4.
ABVS is advantageous as compared with HHUS in that it is less dependent on the examiners, it has an excellent reproducibility, and it can determine the location of lesions more accurately by obtaining the images of the overall breast. Also, it has a shorter examination time than the HHUS. In our study, the average scanning time of ABVS was 9.8±1.3 minutes while the average scanning time of HHUS was 19.6±1.6 minutes (data not shown). However, its use for the axilla examination is limited and therefore the examination of the axilla should be performed additionally. Although the identification of the mass located inferior to the nipple can be done on a 4-scan method where the breast is equally divided into four parts and each nipple includes a scanning [20], there might be variations in the adjustment of artifacts depending on the examiners. To overcome these disadvantages, we perform an overall scanning of the lesions with the help of the radiologic technicians who have been well-trained with HUUS that is additionally installed in ABVS. Then, the images were reviewed by the radiologist during their interpretations. Thus, attempts were made to resolve the problems. The mass located inferior to the nipple is examined using a 4-scan method in a similar manner to other studies. Chang et al. [16] reported that the rate of HHUS mammographically detects occult breast cancers by ABVS were 57.1% to 78.6%, substantial experiences and trainings are necessary to improve cancer detection by ABVS.
There might be a controversy as to the usefulness of breast US as a screening tool. In patients with dense breast, the diagnostic rate of MMG is decreased [2]. It is therefore well-known that the additional use of breast US can raise the diagnostic rate. Particularly in Korea, as compared with Western countries, the proportion of young women with breast cancer is relatively higher and the proportion of dense breast is also relatively higher [21]. According to a study where HHUS was replaced with ABVS, the ABVS was somewhat advantageous as a screening tool as it is not dependent on the examiners and its examination time is relatively shorter [22]. However, our study was not designed to fully answer the questions of breast cancer screening. Thus, if the further study is performed, it is presumed that ABVS deserves special attention as a screening tool.
The present study has several limitations. First, we had no histopathologic data on the benign lesions demonstrated by ABVS and HHUS. So, we could not calculate the sensitivity and specificity of ABVS and HHUS. Second, we did not evaluate the comparison of the images between the ABVS and MMG. Thus, we could not identify the efficacy of ABVS for microcalcifications. Third, we only performed HHUS follow-up and we had no follow-up data on the additional lesions demonstrated by ABVS. These lesions should receive a long-term ABVS follow-up to confirm clinical stability. Lastly, the study was conducted under the retrospective design and enrolled a smaller number of patients, not the screening populations. Thus, our study is a pilot study for a large prospective study. Further well-designed prospective studies are therefore warranted to overcome the demerits of ABVS, as mentioned above, and to examine its usefulness in diagnosis of breast cancer.
In conclusion, there was no significant difference in the characterization of the breast lesions by ABVS and HHUS. In addition, ABVS is also advantageous in that it is not dependent on the examiners, it can visualize the breast within the visual field and it is less time-consuming. It is therefore presumed that it is feasible for clinical applications and is a useful modality in diagnosing breast lesions.
Figures and Tables
Figure 1
Automated breast volume scanner (ABVS) system (ACUSON S2000™; Siemens, Berlin, Germany). (A) The overall view of ABVS system. (B) A 14L5BV transducer (14 MHz, 15.4 cm) that has been designed for the ABVS.

Figure 2
Multiplanar reconstruction of the volume data displayed on automated breast volume scanner. (A) Three on one view images that are provided following a multiplanar reconstruction using a workstation. Coronal view (left), longitudinal (right, upper), and transverse views (right, lower) are synchronously visualized on the screen. (B) A multislice view that has been designed to create serial sections of the images of overall breast in a similar manner to the computed tomography.
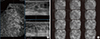
Figure 3
Breast mass on automated breast volume scanner (ABVS) and handheld ultrasound (HHUS). Additionally, 67 lesions were detected on the ABVS and 33 lesions were detected on the HHUS.
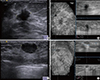
Figure 4
Comparison of automated breast volume scanner (ABVS) and handheld ultrasound (HHUS). A comparison of the images which were interpreted as the lesions of the Breast Imaging Reporting and Data System (BI-RADS) category 4B in HHUS (A) and ABVS (B). This lesion was confirmed to invasive ductal carcinoma by histologic examination. Additionally, a comparison of the images which were interpreted as the lesions of the BI-RADS category 3 in HHUS (C) and ABVS (D).
References
1. Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002; 137(5 Part 1):347–360.

2. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002; 225:165–175.

4. Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009; 192:390–399.

5. Singh S, Tourassi GD, Baker JA, Samei E, Lo JY. Automated breast mass detection in 3D reconstructed tomosynthesis volumes: a featureless approach. Med Phys. 2008; 35:3626–3636.

6. Kotsianos-Hermle D, Hiltawsky KM, Wirth S, Fischer T, Friese K, Reiser M. Analysis of 107 breast lesions with automated 3D ultrasound and comparison with mammography and manual ultrasound. Eur J Radiol. 2009; 71:109–115.

7. Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010; 20:734–742.

8. Maturo VG, Zusmer NR, Gilson AJ, Smoak WM, Janowitz WR, Bear BE, et al. Ultrasound of the whole breast utilizing a dedicated automated breast scanner. Radiology. 1980; 137:457–463.

9. American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. Vol. 2, Breast ultrasound. 4th ed. Reston: American College of Radiology;2003.
10. Zonderland HM, Coerkamp EG, Hermans J, van de Vijver MJ, van Voorthuisen AE. Diagnosis of breast cancer: contribution of US as an adjunct to mammography. Radiology. 1999; 213:413–422.

11. Tozaki M, Fukuma E. Accuracy of determining preoperative cancer extent measured by automated breast ultrasonography. Jpn J Radiol. 2010; 28:771–773.

12. Wang HY, Jiang YX, Zhu QL, Zhang J, Dai Q, Liu H, et al. Differentiation of benign and malignant breast lesions: a comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur J Radiol. 2012; 81:3190–3200.

13. Lin X, Wang J, Han F, Fu J, Li A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol. 2012; 81:873–878.

14. Kim H, Cha JH, Oh HY, Kim HH, Shin HJ, Chae EY. Comparison of conventional and automated breast volume ultrasound in the description and characterization of solid breast masses based on BI-RADS features. Breast Cancer. Epub 2012 Oct 20. http://dx.doi.org/10.1007/s12282-012-0419-1.

15. Wöhrle NK, Hellerhoff K, Notohamiprodjo M, Reiser MF, Clevert DA. Automated breast volume scanner (ABVS): a new approach for breast imaging. Radiologe. 2010; 50:973–981.
16. Chang JM, Moon WK, Cho N, Park JS, Kim SJ. Breast cancers initially detected by hand-held ultrasound: detection performance of radiologists using automated breast ultrasound data. Acta Radiol. 2011; 52:8–14.

17. Lander MR, Tabár L. Automated 3-D breast ultrasound as a promising adjunctive screening tool for examining dense breast tissue. Semin Roentgenol. 2011; 46:302–308.

18. Wojcinski S, Farrokh A, Hille U, Wiskirchen J, Gyapong S, Soliman AA, et al. The automated breast volume scanner (ABVS): initial experiences in lesion detection compared with conventional handheld B-mode ultrasound: a pilot study of 50 cases. Int J Womens Health. 2011; 3:337–346.

19. Tozaki M, Fukuma E. Category assessment based on 3D volume data acquired by automated breast ultrasonography. Jpn J Radiol. 2012; 30:185–191.

20. Isobe S, Tozaki M, Yamaguchi M, Ogawa Y, Homma K, Satomi R, et al. Detectability of breast lesions under the nipple using an automated breast volume scanner: comparison with handheld ultrasonography. Jpn J Radiol. 2011; 29:361–365.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download