Abstract
Purpose
This pilot study aimed to evaluate prognostic factors of postmastectomy radiotherapy (PMRT) for breast cancer patients undergoing systemic therapy in either preoperative or postoperative setting.
Methods
Between 2003 and 2009, 113 patients received PMRT: 61 underwent preoperative systemic therapy (PST subgroup) and 52 received postoperative systemic therapy (non-PST subgroup).
Results
The median follow-up time was 72.3 months (range, 34.0-109.4 months) for surviving patients. In univariate analysis of all patients, disease-free survival (DFS) was associated with age, nodal ratio (NR), and Ki-67 expression; overall survival (OS) was associated with NR and Ki-67 expression. Pathologic N stage and HER2 expression were marginally associated with DFS and OS. In the non-PST subgroup, DFS was associated with age, NR, venous invasion, and Ki-67 expression; OS was associated with age. In the PST subgroup, DFS was associated with ypN stage and NR; OS was associated with ypN, histologic grade, HER2 expression, and p53 expression. In multivariate analysis of all patients, DFS and OS were significantly associated with NR (p=0.003 and p=0.019, respectively) and Ki-67 expression (p=0.002 and p=0.015, respectively). Patients were classified into low-risk (NR ≤0.2 and Ki-67 ≤20%; n=34), intermediate-risk (NR >0.2 or Ki-67 >20%; n=63), and high-risk (NR >0.2 and Ki-67 >20%; n=16) subgroups. All low-risk patients were alive at the time of analysis. High-risk (p<0.001 and p=0.001, respectively) and intermediate-risk (p=0.022 and p=0.008, respectively) patients had significantly shorter DFS and OS than low-risk patients. This prognostic model was statistically significant for DFS when applied to the PST (p=0.001) and non-PST (p=0.016) subgroups separately.
Conclusion
For breast cancer patients undergoing PMRT, NR and Ki-67 are potential prognostic factors. A model using these factors might help predict a poor prognosis. Whether NR and Ki-67 are also prognostic for different setting of systemic therapy, preoperative or postoperative, warrants further study.
For patients with locally advanced breast cancer (LABC), even after mastectomy and systemic therapy, the possibility of occult disease cannot be excluded. Postmastectomy radiotherapy (PMRT) is performed to improve locoregional control (LRC) and survival, a strategy supported by the findings of a number of randomized trials [1-3].
Axillary lymph node (ALN) status is an important prognostic factor for LRC and survival in patients with breast cancer, and the 7th American Joint Committee on Cancer (AJCC) staging system for breast cancer is based on the absolute number of pathologically positive ALNs [4]. Recently, several studies have reported that the nodal ratio (NR), the proportion of involved ALNs amongst all excised ALNs, is of equal prognostic importance [5-10]. In addition, both gross pathologic and biomolecular parameters can be useful prognostic factors for breast cancer. In this regard, hormone receptor (HR) status and c-erbB-2/HER2 status are markers of specific intrinsic subtypes of breast cancer. The Ki-67 index, a marker of cell proliferation, is likewise a marker of a specific intrinsic subtype [11,12] and is also associated with breast cancer recurrence and death [13-16].
Conventionally, PMRT was performed following postoperative systemic therapy in LABC patients. Recently however, preoperative systemic therapy (PST) following PMRT has been widely used in order to facilitate conservation of breast tissue. Here, we report the results of a pilot study designed to identify prognostic or predictive factors for patients with LABC who undergo PMRT in either preoperative or postoperative setting of systemic therapy.
With the approval of the Institutional Review Board of Seoul National University Bundang Hospital (B-1205/153-107), we retrospectively reviewed the medical records of 113 patients with LABC who underwent mastectomy followed by PMRT between March 2003 and December 2009 (Figure 1). Patients who had synchronous metastases at diagnosis, a history of malignancy, or incomplete radiotherapy (RT) were excluded from the present study. The pathologic stage was graded according to the seventh edition of the AJCC cancer staging system [4]. Patient and tumor characteristics are listed in Tables 1 and 2.
All the patients underwent mastectomy. ALN dissection (level I and II) was performed in 110 cases (97%), with sentinel lymph node biopsy alone performed in the remaining 3 (3%). Of the patients undergoing ALN dissection, 70 underwent ALN dissection alone and 40 underwent sentinel lymph node biopsy followed by ALN dissection (Table 3).
The most common PST regimen was DA (docetaxel and doxorubicin) followed by ACT (doxorubicin, cyclophosphamide, and paclitaxel). After completion of PST, the PST subgroup patients underwent mastectomy with ALN dissection. ACT was the most common adjuvant chemotherapy regimen. Adjuvant hormonal therapy was administered in patients with a positive HR status and consisted of 5 years of tamoxifen for premenopausal women and initial aromatase inhibitor therapy or a switch from tamoxifen to aromatase inhibitor therapy for postmenopausal women. Trastuzumab was recommended for all patients with a tumor exhibiting c-erbB-2 overexpression (3+) or HER2 gene amplification (Table 3).
For RT, the chest wall and supraclavicular fossa were irradiated with up to 50.4 Gy at 1.8 Gy per fraction with 5 fractions per week; for a scar boost, 9 Gy at 1.8 Gy per fraction with electrons was administered. Two opposing tangential and one anterior photon beam were used for chest wall and supraclavicular fossa RT, respectively (Table 3). PMRT was started after the completion of adjuvant chemotherapy. When capecitabine was used as the adjuvant chemotherapeutic agent, the patient received PMRT concurrently (n=4).
We reviewed the following histopathologic parameters: estrogen receptor (ER) status; progesterone receptor (PR) status; and the expression of c-erbB-2, p53, Ki-67, and COX-2. Baseline histopathologic parameters were evaluated by immunohistochemical (IHC) analysis using pre-PST biopsy specimens (PST subgroup) or surgical specimens (non-PST subgroup). IHC staining was performed using a BenchMark XT autostainer (Ventana Medical Systems, Tucson, USA) and an i-View detection kit (Ventana Medical Systems) as previously described [17]. The positive cut-off values were IHC staining in ≥1% for ER/PR [18], in >10% for p53, and a 3+ staining score for COX-2 and c-erbB-2. The NR was defined as the number of ALNs with cancer involvement divided by the total number of excised ALNs. Lymph node (LN) status was evaluated by hematoxylin and eosin staining. Fluorescence in situ hybridization was performed for the analysis of HER2 gene amplification as reported previously [17].
The base follow-up duration was defined from the date when the first treatment was initiated. In cases of treatment failure, we analyzed the first site of relapse. Locoregional recurrence (LRR) included recurrences in the ipsilateral chest wall or ipsilateral regional LNs (axillary, supra/infraclavicular, and internal mammary). Relapses in the contralateral chest wall, axillary LNs, supra/infraclavicular LNs, internal mammary LNs, cervical LNs, or other organs were defined as distant metastases (DM).
Using the Kaplan-Meier method and the log-rank test, survival curves and differences between subgroups were estimated. For multivariate analysis, the Cox proportional hazards method was used. To compare proportions between subgroups, Pearson chi-square and Fisher exact test were used. SPSS version 18.0 (SPSS, Chicago, USA) was used for statistical analyses. A p-value less than 0.05 was deemed to be statistically significant.
Generally, a value above 10% to 20% of the Ki-67 index was defined as a high level [12-14,16]. We compared survival curves using 3 hypothetical cut-off values, 10%, 15%, and 20% of the baseline Ki-67 index, and found that the latter gave the most significant differences.
The NR cut-off value used in previous studies varied from 0.15 to 0.25 [5,7-10]. We used 6 candidates for the cut-off value of the NR, ranging from 0.05 to 0.3 with intervals of 0.05. The maximal chi-square method in the R program version 2.13.0 (R Development Core Team, Vienna, Austria; available from http://www.R-project.org) was used to obtain the optimal cut-off value of the NR, which was 0.2.
A total of 61 patients with an advanced clinical T stage tumor (T3 and T4) or ALN involvement received PST. In the PST subgroup, 7 patients received additional chemotherapy because of adverse pathologic features such as advanced stage or negative HR status. The other 52 patients received adjuvant systemic therapy. Chest wall and supraclavicular fossa irradiation was administered in 106 patients, and chest wall irradiation only in 7 patients. A total of 3 patients received a scar boost. The median number of excised ALNs was 22 (range, 1-55) in the whole cohort and 23 (range, 1-55) and 21 (range, 5-50) in the non-PST and PST subgroups, respectively. The median NR was 0.19 (range, 0-1) in the whole cohort, including patients with pathologically noninvolved ALNs (pN0), and 0.26 (range, 0.03-1.0) in patients with pathologically involved ALNs (pN+). We used the NR of 0.2 as a cut-off value to classify patients into high and low NR groups.
The median follow-up duration was 72.3 months (range, 34.0-109.4 months) for surviving patients. In the entire cohort, the 5-year survival rates were 87.2%, 78.9%, 77.3%, and 85.3% for locoregional progression-free survival (LRPFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS), respectively.
With respect to the type of initial disease relapse, LRR occurred in 4 patients (PST subgroup, 4), DM in 14 patients (non-PST subgroup, 7; PST subgroup, 7), and both LRR and DM in 10 patients (non-PST subgroup, 3; PST subgroup, 7). One of the patients with initial LRR underwent resection and the other 3 underwent systemic therapy. Of those patients with initial LRR and DM, 1 patient underwent resection and systemic therapy, 1 patient underwent chemotherapy and whole brain irradiation, and 6 patients were treated using systemic therapy only.
Univariate analysis revealed that patients with a NR of >0.2 had a significantly lower DMFS (p=0.003), DFS (p=0.006), and OS (p=0.032) than those with a NR of ≤0.2. Patients with a baseline Ki-67 index of >20% had a significantly lower LRPFS (p=0.032), DMFS (p=0.013), DFS (p=0.007), and OS (p=0.030) than those with a baseline Ki-67 index of ≤20%. The baseline HR level was associated with LRPFS (p=0.025) but not with DMFS (p=0.379), DFS (p=0.236), and OS (p=0.253). The pathologic nodal stage was marginally associated with DMFS (p=0.064), DFS (p=0.087), and OS (p=0.084). These results are detailed in Table 4, Figures 2 and 3.
We also performed subgroup analysis for the non-PST and PST subgroups. In the former, age (p=0.010), NR (p=0.030), venous invasion (p=0.035), and the baseline Ki-67 index (p=0.037) were associated with DFS, although only age (p=0.048) was associated with OS. In the PST subgroup, ypN stage (p=0.048) and NR (p=0.028) were associated with DFS and ypN (p=0.030), histologic grade (p<0.001), baseline HER2 expression (p=0.048), and baseline p53 expression (p=0.026) were associated with OS (Table 5).
We performed multivariate analysis incorporating the NR, baseline Ki-67 index, age, histologic grade, and baseline p53 expression, all of which were found to be significantly associated with DFS or OS in univariate analysis of the entire cohort. A high NR was associated with poor DMFS (relative risk [RR], 4.063; 95% confidence interval [CI], 1.701-9.701; p=0.002), DFS (RR, 3.589; 95% CI, 1.567-8.220; p=0.003), and OS (RR, 3.444; 95% CI, 1.227-9.669; p=0.019). A high baseline Ki-67 index was associated with poor DMFS (RR, 3.125; 95% CI, 1.450-6.731; p=0.004), DFS (RR, 3.274; 95% CI, 1.536-6.979; p=0.002), and OS (RR, 3.133; 95% CI, 1.249-7.856; p=0.015). Results of the multivariate analysis are detailed in Table 6.
We devised a prognostic model using the NR and baseline Ki-67 index, with a score of zero points for a NR of ≤0.2 or a baseline Ki-67 index of ≤20% and 1 point for a NR of >0.2 or a baseline Ki-67 index of >20%. Patients were classified into 3 subgroups according to their total score: low risk (0 point, n=34), intermediate risk (1 point, n=63), and high risk (2 points, n=16). No deaths occurred in the low-risk group, whereas 13 patients in the intermediate-risk group and 6 patients in the high-risk group had died at the time of the last follow-up. When comparing the high- and low-risk patients, a significant difference was found in LRPFS (p=0.040), DMFS (p<0.001), DFS (p<0.001), and OS (p<0.001). A significant difference was also observed between the intermediate- and low-risk groups with respect to DMFS (p=0.031), DFS (p=0.022), and OS (p=0.008), but not LRPFS (p=0.204) (Figure 4).
We used the Cox proportional hazards method in order to evaluate the RR among the different risk groups. For LRPFS, the high- and intermediate-risk groups demonstrated RRs of 4.898 (95% CI, 0.897-26.753; p=0.067) and 2.599 (95% CI, 0.562-12.032; p=0.222), respectively, and for DMFS, the RRs of the high- and intermediate-risk groups were 14.110 (95% CI, 3.089-64.448; p=0.001) and 4.400 (95% CI, 1.006-19.241; p=0.049), respectively. With respect to DFS, the high- and intermediate-risk patients showed RRs of 14.264 (95% CI, 3.122-65.165; p=0.001) and 4.785 (95% CI, 1.100-20.814; p=0.037), respectively.
We applied this prognostic model to the non-PST and PST subgroups. There was a significant difference in DMFS (p=0.016 and p<0.001) and DFS (p=0.016 and p<0.001) in the non-PST and PST subgroups, respectively. There was no significant difference in the LRPFS in the non-PST (p=0.364) and PST (p=0.224) subgroups, although there was a significant difference with respect to OS in the PST subgroup (p=0.045) but not in the non-PST subgroup (p=0.074).
Several large, randomized studies have shown that PMRT improves LRC and survival in breast cancer patients, particularly those with more than 3 involved ALNs [1-3]. To date though, the role of PMRT in breast cancer patients with fewer than 4 metastatic ALNs has not been evaluated. Overgaard et al. [19] conducted a reanalysis of Danish trials and found that PMRT benefited patients with 1 to 3 positive ALNs. Recently, the number of excised ALNs was shown to be as important as the number of involved ALNs, suggesting that the NR is an important prognostic factor [5-10]. In a study by the Surveillance, Epidemiology and End Results program, the NR was found to be better at predicting disease-specific survival than the number of involved ALNs [7]. Truong et al. [8] reported that an NR of 0.25 was associated with a poor prognosis with respect to LRR, DM, and OS in patients with 1 to 3 involved ALNs. Ahn et al. [10] analyzed a nationwide registry of pN+ patients and concluded that the NR was a better prognostic factor than pN stage, particularly in patients with high-risk factors such as young age, a HER2/neu-enriched tumor, or a triple-negative tumor.
In the study we report here, the pN stage showed only a borderline association with recurrence or survival in univariate analysis. A possible reason for this finding may be the heterogeneity in the sequence of systemic therapy. After PST, more patients could have a lower N stage as a result of chemotherapy. This finding might also be explained by the relatively small size and short follow-up period of our study. As shown in Figure 2, the DFS and OS curves differed between patients with pN0-1 and pN2-3, and it is possible that with a greater number of patients and a longer follow-up period, a statistically significant relationship might be found between survival and pN stage.
Although this pilot study included patients with different pN stages, the NR (cut-off value of 0.2) was associated with a high risk of metastasis and short survival in LABC patients. Because the NR reflects the absolute number of excised ALNs, it might have a higher prognostic value than pN stage [20]. In the current cancer staging system [4], the usefulness of the absolute number of involved nodes for predicting disease burden in the axilla is confounded by the number of nodes removed [21]. When additional ALNs are excised, less residual occult disease may be expected. In Canada, ALN dissection, including all level I and II ALNs, is recommended for accurate staging and reducing the risk of recurrence in the axilla [22].
Although several studies have reported a possible prognostic role for the NR in LRC [8,23,24], we could not establish a relationship between the NR and LRC in this study. This may have been because of the relatively short follow-up duration (approximately 6 years). Improved LRC as a result of regional RT [25,26] might also account for the lack of any significant difference in LRPFS between the high NR and low NR patient groups. We suggest therefore that a randomized controlled study focusing specifically on the prognostic role of NR be conducted.
In addition to the NR, biomolecular markers might also have prognostic value for LABC patients. It is generally accepted that biomolecular markers of cell proliferation, such as the baseline Ki-67 index used in our study, are associated with the response to systemic therapy [27]. Our study showed that a high baseline Ki-67 index was associated with a high risk of mortality. Furthermore, a relationship between a high Ki-67 index and other indicators of a poor prognosis has been previously reported [28]. This negative relationship would explain the prognostic value of Ki-67 index. In the present study, a Ki-67 index in excess of 20% was associated with baseline negative HR expression (p<0.001).
Consistent with our findings, the Ki-67 index has been shown to be a possible prognostic marker in several other studies. Cheang et al. [12] classified invasive breast cancer into luminal A, luminal B, and HER2-positive intrinsic subtypes on the basis of hormone receptor status, HER2 status, and the Ki-67 index, as determined using IHC. The Ki-67 index was used to distinguish luminal B from luminal A, using a cut-off value of 14%. The luminal B and luminal HER2 subtypes were found to have a poor prognosis with respect to breast cancer recurrence-free and disease-specific survival. The 10-year breast cancer-specific survival rates were 92%, 79%, and 78% in luminal A, luminal B, and HER2 positive cancer, respectively (p<0.001). In a meta-analysis study of early breast cancer, Ki-67 positivity (cut-off points were defined by the authors of the studies being included) was associated with increased relapse (RR, 1.93; 95% CI, 1.74-2.14; p<0.001) and shorter survival (RR, 1.95; 95% CI, 1.70-2.24; p<0.001) in all patients. The authors of that study suggested that Ki-67 positivity was a prognostic marker in patients with early breast cancer [14].
Despite these studies, in general, the association between specific biomolecular markers and LRC remains unclear. Two previous studies found that the Ki-67 index was a possible prognostic factor of LRC [13,16]. Voduc et al. [13] defined the luminal B subtype as being ER(+) or PR(+) and HER2(-) and having a Ki-67 index of ≥14% in patients who had undergone mastectomy. The luminal B subgroup was associated with a high risk of local and regional recurrences. Selz et al. [16] reported that a Ki-67 index of >20% was prognostic for LRFPS (RR, 4.18; 95% CI, 1.11-15.77; p=0.0215) in breast cancer patients with pN0 after modified radical mastectomy.
In addition to HER2 status, the Ki-67 index, representing tumor aggressiveness, may also be a means of identifying high-risk groups among breast cancer patients. However, controversy still exists regarding the optimal cut-off point for Ki-67; a level of Ki-67 above 10% to 20% has been suggested to define a high-risk group in several studies [12-14,16]. In our study, the baseline Ki-67 index was used to determine the risk groups, using a cut-off point of 20%. The 2011 St. Gallen Consensus [11] recommended a Ki-67 labeling index of 14% as the cut-off point to classify the intrinsic subtype of breast cancer; however, these guidelines have not been clarified. It therefore remains necessary to develop a standardized approach to using the Ki-67 index, including a single cut-off value and a reproducible way of determining the index.
Here, we propose a prognostic model using 2 parameters, the NR and baseline Ki-67 index, both of which are significantly associated with disease relapse, reflecting the probability of residual tumor on a macroscopic scale and the possibility of disease relapse on a microscopic scale, respectively. Our prognostic model is simple to apply and can identify the poor prognostic group amongst a heterogeneous population with disparate pN stages or sequences of systemic therapy. Using our prognostic model, patients with a high risk of disease relapse can be identified, and intensified adjuvant treatment can be considered to improve their survival. With respect to LRC, however, the high-risk group tended to have a worse prognosis than the low-risk group (p=0.067), and the intermediate-risk group showed no association. We expect that this prognostic model would be more useful to identify the high-risk group among LABC patients with an increased long-term follow-up period.
Our study has several limitations. The patients needed to be analyzed independently according to the use of PST because the NR has a prognostic value in patients with PST [6]. However, subgroup multivariate analysis was not performed because of an insufficient number of patients. In addition, the relatively short follow-up duration was a hindrance to comparing OS. These limitations may have made it more difficult to identify a relationship between treatment outcomes and well-known prognostic factors, such as T/N stage and HR status. Furthermore, the study included patients with a range of different N stages and 21% of patients had pN0 tumors (21%), whereas the other studies on the prognostic value of NR discussed here only involved node-positive patients. Therefore, a further study is needed with a more homogenous patient group with respect to the sequence of systemic therapy and pN stage. Additionally, for a more precise prognostic model, the change in biomarker status before and after PST [15,29] should be considered.
In conclusion, we found that the NR and baseline Ki-67 index were potential prognostic markers in LABC patients who underwent PMRT. Our prognostic model, using these 2 factors, might be able to identify patients at high risk of disease relapse. Improved prognostic models will help to individualize treatment regimens for breast cancer patients.
Figures and Tables
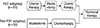 | Figure 1A flow sheet on treatment of breast cancer: preoperative systemic therapy (PST) was considered in patients with advanced clinical T stage or axillary lymph node involvement.
*Chemotherapy was administered before and after mastectomy; or additional chemotherapy was given in patients with adverse pathologic features.
|
 | Figure 2Survival curves in the patients with breast cancer having postmastectomy radiotherapy: locoregional progression-free survival according to the nodal ratio (A) and the baseline Ki-67 (B); disease-free survival according to the nodal ratio (C) and the baseline Ki-67 (D); overall survival according to the nodal ratio (E) and the baseline Ki-67 (F). |
 | Figure 3Survival curves in the patients with breast cancer having postmastectomy radiotherapy according to pathologic nodal stage: (A) disease-free survival and (B) overall survival. |
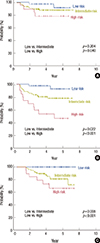 | Figure 4Survival curves in the patients with breast cancer having postmastectomy radiotherapy according to the risk group: (A) locoregional progression-free survival, (B) disease-free survival, and (C) overall survival. |
Notes
References
1. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997; 337:949–955.

2. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999; 353:1641–1648.

3. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005; 97:116–126.

4. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York: Springer;2010.
5. Han TJ, Kang EY, Jeon W, Kim SW, Kim JH, Kim YJ, et al. The prognostic value of the nodal ratio in N1 breast cancer. Radiat Oncol. 2011; 6:131.

6. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009; 116:153–160.

7. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009; 27:1062–1068.

8. Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005; 103:2006–2014.

9. Truong PT, Woodward WA, Thames HD, Ragaz J, Olivotto IA, Buchholz TA. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1-3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2007; 68:59–65.

10. Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean Breast Cancer Society. Breast Cancer Res Treat. 2011; 130:507–515.

11. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.

12. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750.

13. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–1691.

14. de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007; 96:1504–1513.

15. Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009; 116:53–68.

16. Selz J, Stevens D, Jouanneau L, Labib A, Le Scodan R. Prognostic value of molecular subtypes, Ki67 expression and impact of postmastectomy radiation therapy in breast cancer patients with negative lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2012; 84:1123–1132.

17. Jang MH, Kim EJ, Choi Y, Lee HE, Kim YJ, Kim JH, et al. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012; 14:R115.
18. Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol. 2011; 29:e458.

19. Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007; 82:247–253.

20. Schmoor C, Sauerbrei W, Bastert G, Bojar H, Schumacher M. German Breast Cancer Study Group. Long-term prognosis of breast cancer patients with 10 or more positive lymph nodes treated with CMF. Eur J Cancer. 2001; 37:1123–1131.

21. Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006; 24:2910–2916.

22. Canadian Association of Radiation Oncologists; The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Axillary dissection. CMAJ. 1998; 158:Suppl 3. S22–S26.
23. Katz A, Buchholz TA, Thames H, Smith CD, McNeese MD, Theriault R, et al. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: implications for adjuvant irradiation. Int J Radiat Oncol Biol Phys. 2001; 50:397–403.

24. Grills IS, Kestin LL, Goldstein N, Mitchell C, Martinez A, Ingold J, et al. Risk factors for regional nodal failure after breast-conserving therapy: regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys. 2003; 56:658–670.

25. Wai ES, Lesperance M, Speers CH, Truong PT, Jones S, Tyldesley S, et al. Increased use of regional radiotherapy is associated with improved outcome in a population-based cohort of women with breast cancer with 1-3 positive nodes. Radiother Oncol. 2010; 97:301–306.

26. Truong PT, Jones SO, Kader HA, Wai ES, Speers CH, Alexander AS, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009; 73:357–364.

27. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004; 351:2817–2826.

28. Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008; 26:5569–5575.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download