Abstract
Purpose
Preclinical studies have shown that human epidermal growth factor receptor 2 (HER2) status is associated with resistance to radiotherapy (RT). In this study, we evaluated the overall survival of a T1N0M0 breast cancer cohort in Korea according to the use of RT and the HER2 status.
Methods
We analyzed data collected from 11,552 patients with invasive breast cancer who were enrolled in the Korean Breast Cancer Society Registration Program between 1999 and 2007. Data on the TNM stage, estrogen receptor status, progesterone receptor status, HER2 status, operation method, and the use of RT were analyzed.
Results
The median follow-up period was 51 months. A significant improvement in overall survival after RT was observed only in the HER2(-) group. In this group, the 10-year overall survival rate was 95.5% for patients who did not receive RT and 96.3% for patients who received RT (p=0.037). In contrast, in the HER2(+) group, RT was not associated with a survival benefit (p=0.887). Multivariate analysis showed that RT was significantly associated with a reduction in mortality in the HER2(-) group (hazard ratio, 0.738; 95% confidence interval, 0.549-0.993; p=0.045).
Radiotherapy (RT) is an important treatment modality in breast cancer management. Multiple clinical trials have demonstrated long-term RT-related mortality benefits in patients with breast cancer [1-4]. The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) performed a meta-analysis to determine the effects of RT after breast-conserving surgery (BCS). In this analysis, RT reduced the 15-year risk of breast cancer death from 25.2% to 21.4%, an absolute reduction of 3.8% (95% confidence interval [CI], 1.6-6.0; p=0.00005) [4]. Several important trials have evaluated the effects of postmastectomy RT. The Danish Breast Cancer Cooperative Group (DBCG) protocols 82b and 82c, the British Columbia trial, and the EBCTCG meta-analysis showed that postmastectomy RT was associated with a significant improvement in overall survival [1,3,5,6]. Taken together, these results suggest that RT-induced locoregional control can result in improved overall survival.
The human epidermal growth factor receptor 2 (HER2) is amplified or overexpressed in 20% to 25% of patients with breast cancer [7,8]. Recent reports suggest that HER2 expression is associated with a high rate of locoregional recurrence [9-11]. Although the relationship between HER2 status and treatment failure is generally accepted, the exact mechanism is not entirely understood. Some preclinical studies showed that HER2 plays a major role in the modulation of radiation sensitivity [12-14]. Breast cancer cells develop resistance to RT when transfected with the HER2 gene [12,14]. Further, specific inhibition of HER2 mRNA by siRNA increased the radiosensitivity of HER2(+) SKBR3 breast cancer cells [13]. However, few clinical studies on this subject have been reported. The DBCG conducted a subgroup analysis of overall survival according to molecular subtype in high-risk patients who participated in trials based on the DBCG protocols 82b or 82c [15]. In this analysis, patients with hormone receptor (HR)(-)/HER2(+) status did not show any survival improvement after treatment with total mastectomy plus partial axillary dissection and RT. Although the participants were high-risk patients, this analysis suggested that HER2(+) tumors are possibly resistant to RT.
To evaluate the effects of RT on survival according to the HER2 status in early breast cancer patients, we retrospectively analyzed data from the nationwide Korean breast cancer patient cohort.
The Korean Breast Cancer Society (KBCS) registry database was used to identify node-negative patients with newly diagnosed invasive breast cancer with a tumor less than ≤2 cm between January 1999 and December 2007. The KBCS registry has prospectively collected nationwide breast cancer data since 1996 [16-19]. The Online Korean Breast Cancer Registration Program was launched in 2001. Physicians personally enter clinicopathological data of newly diagnosed biopsy-proven primary breast cancer patients into the web-based database. The database contains information regarding the patients' sex and age, the surgical method used, the histologic findings, status of biologic markers (including HER2 status), adjuvant treatment, and cancer stage (according to the sixth American Joint Committee on Cancer classification). Patient survival data, including the dates and causes of death, were obtained from the Death Certification of the Korean National Statistical Office and the Korean Central Cancer Registry of the Ministry of Health and Welfare. The Korean Central Cancer Registry is linked to the Korea National Statistical Office, which has recorded complete death statistics by using a unique identification numbers that are assigned to all Korean residents [16-19]. Between January 1999 and December 2007, 52,185 patients were registered in this database. The database includes more than 40% of the incidence data from The Korea National Cancer Incidence Database (KNCID), and the completeness of the KNCID for 2009 was 97.2%, as determined by the Ajiki method [20]. Detailed information on the KBCS registry has been provided elsewhere [16-20]. The last date of follow-up was December 31, 2008. This study was approved by the Institutional Review Board of the Korea Cancer Center Hospital (approval number: K-1305-002-017).
Of the 52,185 patients identified, we excluded those patients with incomplete information regarding estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, operation method, and use of RT and male patients. Patients with bilateral disease, malignant phyllodes tumor, lymphoma, sarcoma, squamous cell carcinoma, prior malignancy, and prior treatment with neoadjuvant chemotherapy were also excluded. Thus, a total of 11,552 patients were included in this analysis (Figure 1).
HER2 positivity was defined as a rating of 3+ on immunohistochemistry (IHC) and/or gene amplification on fluorescence in situ hybridization (FISH). ER and PR status was defined as positive or negative by physicians according to each institution's standard methods and cutoff values. Tumors that were ER or PR positive were defined as hormone receptor (HR) positive, and tumors that were ER and PR negative were defined as HR negative.
Data analysis was performed using SPSS version 14.0 (SPSS Inc., Chicago, USA). The chi-square test was used to assess differences in the clinicopathological factors between the groups. Survival rates were estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. The Cox proportional hazards model was used to perform multivariate analyses. Statistical significance was accepted for p-values of less than 0.05.
Patient and tumor characteristics and data on the adjuvant systemic treatment received are summarized in Table 1. The mean age of the patients was 49.0±10.1 years, and 6,646 patients (57.5%) were younger than 50 years. Of the 11,552 patients, 18.6% had HER2(+) disease and 72.3% had HR(+) disease. RT had been administered to 6,781 patients (58.7%). The proportion of patients who had undergone BCS was 61.5%, and 92.6% of these patients had received postoperative RT. Among the patients who had undergone mastectomy, only 201 (4.5%) had received postoperative RT. Further, 57.9% of the patients had received adjuvant chemotherapy, and 67.5% had received endocrine therapy. Five patients (0.2%) with HER2(+) tumors had been treated with adjuvant trastuzumab therapy. No patients had received adjuvant lapatinib therapy.
Table 2 summarizes the clinicopathological characteristics according to the use of RT and HER2 status. In the HER2(-) group, 61.5% of the patients had received RT. The HER2(-)/RT(+) group had a larger proportion of patients younger than 50 years (P<0.001), a larger number of patients with HR(-) disease (p=0.028), and fewer patients who were treated with adjuvant chemotherapy than the HER2(-)/RT(-) group. In the HER2(+) group, 943 patients (43.8%) had received RT. The HER2(+)/RT(-) group had a larger proportion of younger patients (p=0.004), fewer patients with HR(-) disease, and a higher frequency of treatment with adjuvant chemotherapy than the HER2(+)/RT(+) group. Operation methods according to RT were significantly imbalanced in both the HER2(-) and HER2(+) groups.
A total of 181 death events occurred during a median follow-up period of 51 months (range, 1-140 months). The 10-year overall survival rate was 96.0% (Figure 2A). Overall survival did not differ significantly between patients with HER2(+) tumors and those with HER2(-) tumors (p=0.208), as shown in Figure 2B. The operation method used was not associated with overall survival (p=0.201) (Figure 2C). In contrast, overall survival differed significantly between patients treated with RT and those not treated with RT. The patients treated with RT had a significantly better overall survival (p=0.040) (Figure 2D).
Among patients with HER2(-) disease, the 10-year overall survival rate was higher in those younger than 50 years (96.5% vs. 95.4%, p=0.029), in those with HR(+) disease (97.1% vs. 92.6%, P<0.001), and in those treated with RT (96.3% vs. 95.5%, p=0.037). No significant difference in overall survival was found between patients treated with adjuvant chemotherapy and those treated without adjuvant chemotherapy.
In contrast, among HER2(+) patients, no significant difference was found among patients with regard to various risk factors, including the age at diagnosis, HR status, adjuvant chemotherapy, and the use of RT. The 10-year overall survival rate in the HER2(+) patient group was 95.7% for patients who did not receive RT and 95.3% for those who received RT (p=0.887) (Table 3, Figure 3).
Overall survival was analyzed according to the molecular subtype and RT use. Among patients with HR(+)/HER2(-) disease, the 10-year overall survival rate was greater in those treated with RT than in those not treated with RT (97.6% vs. 96.5%, p=0.021). However, no significant difference was found in overall survival between patients treated with RT and those treated without RT among patients with HR(+)/HER2 (+) disease (93.9% vs. 96.8%, p=0.182), HR(-)/HER2(+) disease (97.8% vs. 94.6%, p=0.087), or HR(-)/HER2(-) disease (93.2% vs. 92.0%, p=0.372).
To identify the factors that predict overall survival in the subgroup of patients with HER2(-) tumors, multivariate analysis was performed. Variables that were statistically significant in univariate analysis were included in multivariate analysis. These were age at diagnosis, HR status, mass size, and use of RT. Multivariate analysis showed that the use of RT could predict overall survival in patients with HER2(-) disease (hazard ratio [HR], 0.738; 95% CI, 0.549-0.993; p=0.045) (Table 4). In addition, an age of ≥50 years at diagnosis (HR, 1.410; 95% CI, 1.044-1.904; p=0.025) and HR(+) status (HR, 0.351; 95% CI, 0.260-0.472; P<0.001) retained statistical significance as predictors of overall survival. In the HER2(+) group, no factors were found to significantly predict overall survival in univariate analysis. Therefore, multivariate analysis was not performed for this group.
In the present study, we showed that breast cancer patients with HER2(+) disease do not obtain an overall survival benefit from postoperative RT, unlike those with HER2(-) disease. To our knowledge, this is the first large-scale clinical analysis to show that HER2(+) tumors display a tendency for RT resistance in patients with T1N0M0 breast cancer. This result has clinical relevance because the use of RT after BCS is increasing with the trend in the general use of screening mammography.
Although some preclinical data support the hypothesis that the HER2 status is positively associated with RT resistance, the available clinical data on this subject are limited. The DBCG conducted a subgroup analysis of 1,000 patients from the DBCG studies based on protocols 82b and 82c, in which the inclusion criteria were positive lymph nodes and/or tumors of >5 cm in size and/or tumor invasion of the skin or pectoralis muscle fascia [15]. All patients had received total mastectomy, axillary dissection, and 8 cycles of CMF (cyclophosphamide, methotrexate, and fluorouracil) as adjuvant chemotherapy. In subgroup analysis, IHC staining was used to evaluate the ER, PR, and HER2 statuses, and tissue microarrays were used for FISH analyses of HER2 expression. The median follow-up time was 17 years. The 4 subgroups were defined according to ER, PR, and HER2 expression: HR(+)/HER2(-), HR(+)/HER2(+), HR(-)/HER2(-), and HR(-)/HER2(+). Postmastectomy RT did not improve overall survival for the HR(-)/HER2(+) subtype, whereas it significantly improved overall survival for the HR(+)/HER2(-) subtype. In the present nationwide analysis of T1N0M0 patients, we found that no RT-related survival benefits were observed in the HER2(+) group, whereas RT conferred survival benefits in the HER2(-) group. This result closely agrees with those of the DBCG subgroup analyses based on the 82b and 82c protocols, although these analyses were conducted on very different populations. In contrast to the present study and the DBCG subgroup analyses based on the 82b and 82c protocols, Rozan et al. [21] reported that HER2 overexpression was not associated with RT use in a neoadjuvant setting. The authors evaluated tumor responses to FAC (5-fluorouracil, doxorubicin, and cyclophosphamide) chemotherapy or RT. Of 329 patients, 156 were assigned to the neoadjuvant RT group, in which HER2 expression was not significantly associated with tumor response. However, the small number of patients limits the utility of these results; only 26 patients (16.7%) had tumors that were strongly positive for HER2.
Previous reports have suggested that breast cancer subtypes were associated with the risk of local and regional recurrence or survival [4,9,11,22,23]. However, the mechanism by which HER2 positivity predicts a poor prognosis is unknown. To evaluate the relationship between the use of RT and survival, the patients evaluated must not have pre-existing distant micrometastatic disease [24]. Therefore, the target population should include patients with very early-stage disease. In the present study, only patients with T1N0M0 disease were included in the analysis. Despite the relatively short follow-up period and the generally low number of death events in this population, the survival rate differed significantly between the HER2(-)/RT(+) and HER2(-)/RT(-) groups.
The study presented herein was a retrospective analysis, and therefore, it has several limitations. First, a clinical and pathological imbalance existed between the groups. Second, the operation method was excluded from multivariate analysis because it was an intervening variable of RT. However, it is well established that the operation method does not affect the overall survival of patients with early breast cancer. Veronesi et al. [25] found that after 20 years of follow-up, the long-term survival rate of patients who underwent BCS did not differ from that of patients who underwent radical mastectomy. In the National Surgical Adjuvant Breast and Bowel Project B-06, 1,851 patients were assigned randomly to one of several treatment groups: total mastectomy, lumpectomy only, or lumpectomy, and breast irradiation. The findings reported after 20 years showed no significant differences between the groups with regard to disease-free survival, distant disease-free survival, and overall survival [26]. In accordance with these prospective randomized trials, the data from the present study also showed that overall survival was not affected by the operation method (p=0.201). A third limitation of our study was that locoregional recurrence was not analyzed. Because the recurrence data were entered freely by the physicians at each institute, data were incomplete in several cases. In contrast with the recurrence data, the Korean Breast Cancer Society online registry database contains complete and accurate data on death events that are obtained biannually from the Korean National Statistical Office and the Korean Central Cancer Registry of the Ministry of Health and Welfare according to unique identification numbers. Finally, according to the EBCTCG meta-analysis, on average, approximately 1 breast cancer death was avoided by year 15 for every 4 recurrences that were avoided by year 10 as a result of RT [4]. Thus, the median follow-up time of 51 months in our study is a relatively short time with which to provide definitive answers regarding the absolute survival benefits of RT.
Our data showed that HER2 positivity was related to RT resistance in patients with T1N0M0 breast cancer. These results suggest that HER2-targeted therapies are potentially useful as radiosensitizers for patients with HER2(+) tumors. However, the relatively short follow-up period and lack of locoregional recurrence data should be considered when interpreting these findings. In a preclinical study, RT resistance induced by HER2 gene transfection was overcome by trastuzumab both in in vitro and in vivo studies [12]. Inhibition of PI3K-Akt-mTOR signaling (HER2 pathway) has been shown to increase radiosensitivity in HER2(+) breast cancer cell lines [13,27]. In addition, lapatinib, a small molecule tyrosine kinase inhibitor of HER1 and HER2, acts as a radiosensitizer in HER2(+) breast cancer xenografts [28]. Unfortunately, clinical information regarding the radiosensitization effects of HER2-targeted therapy is currently unavailable. At present, trastuzumab and lapatinib are commonly used in clinical practice as standard therapies for the treatment of HER2(+) breast cancer. However, the optimal sequence for RT and trastuzumab in an adjuvant setting for breast cancer treatment is not yet established. Phase II trials showed that concurrent therapy with trastuzumab and RT was well tolerated [29,30]. A large prospective randomized clinical trial might provide insight into whether the combination of RT and HER2-targeted therapy could improve the survival of patients with HER2(+) tumors.
Figures and Tables
Figure 1
Patient selection flow chart.
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.

Figure 2
(A) Overall survival for all patients. (B) Overall survival according to human epidermal growth factor receptor 2 (HER2) status. (C) Overall survival according to operation methods. (D) Overall survival according to whether radiotherapy (RT) or not.
BCS=breast-conserving surgery.

Figure 3
Effect of radiotherapy (RT) on breast cancer mortality after curative operation (A) in the human epidermal growth receptor 2 (HER2) (-) group and (B) in the HER2(+) group.
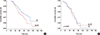
References
1. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999; 353:1641–1648.

2. Danish Breast Cancer Cooperative Group. Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006; 24:2268–2275.

3. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997; 337:949–955.

4. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011; 378:1707–1716.

5. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005; 97:116–126.

6. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–2106.

7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182.

8. Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004; 5:63–69.

9. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–1691.

10. Millar EK, Graham PH, O'Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009; 27:4701–4708.

11. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373–2378.

12. Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999; 59:1347–1355.
13. No M, Choi EJ, Kim IA. Targeting HER2 signaling pathway for radiosensitization: alternative strategy for therapeutic resistance. Cancer Biol Ther. 2009; 8:2351–2361.

14. Pirollo KF, Tong YA, Villegas Z, Chen Y, Chang EH. Oncogene-transformed NIH 3T3 cells display radiation resistance levels indicative of a signal transduction pathway leading to the radiation-resistant phenotype. Radiat Res. 1993; 135:234–243.

15. Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008; 26:1419–1426.

16. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368.

17. Lee JA, Kim KI, Bae JW, Jung YH, An H, Lee ES, et al. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010; 123:177–187.

18. The Korean Breast Cancer Society. Survival analysis of Korean breast cancer patients diagnosed between 1993 and 2002 in Korea: a nationwide study of the cancer registry. J Breast Cancer. 2006; 9:214–229.
19. Han W, Kang SY. Korean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010; 119:193–200.

20. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012; 44:11–24.

21. Rozan S, Vincent-Salomon A, Zafrani B, Validire P, De Cremoux P, Bernoux A, et al. No significant predictive value of c-erbB-2 or p53 expression regarding sensitivity to primary chemotherapy or radiotherapy in breast cancer. Int J Cancer. 1998; 79:27–33.

22. Albert JM, Gonzalez-Angulo AM, Guray M, Sahin A, Strom EA, Tereffe W, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 2010; 77:1296–1302.

23. Wang Y, Yin Q, Yu Q, Zhang J, Liu Z, Wang S, et al. A retrospective study of breast cancer subtypes: the risk of relapse and the relations with treatments. Breast Cancer Res Treat. 2011; 130:489–498.

25. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.

26. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.

27. Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003; 2:1113–1120.
28. Sambade MJ, Kimple RJ, Camp JT, Peters E, Livasy CA, Sartor CI, et al. Lapatinib in combination with radiation diminishes tumor regrowth in HER2+ and basal-like/EGFR+ breast tumor xenografts. Int J Radiat Oncol Biol Phys. 2010; 77:575–581.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download