Abstract
Purpose
Evaluating the effect of hormonal treatment on quality of life (QoL) in breast cancer patients by using the Functional Assessment of Cancer Treatment (FACT) questionnaire is the main purpose of this trial.
Methods
Breast cancer patients treated with adjuvant between January 2007 and December 2009 were evaluated. The first survey was done after patients completed their whole adjuvant treatment except for the hormonal therapy and this was as 'basal assessment.' The second survey was done 6 to 12 months after the basal surveys during their routine policlinic controls. The last survey was done within the last 18 to 24 months of the follow-up period.
Results
The effect of marital status, number of pregnancies, residence in the village or city, hemoglobin levels, chemotherapy and hormonal therapy for any other reason except for breast cancer on the QoL could not be seen. Endocrine subscale scores were detected to be higher in patients aged >60 years than in younger ones. The other dimension scores were low in the elderly patient group. There was a statistically significant relationship between being >30 years old and improvement in the social well-being score (p=0.028). The functional well-being scores were found to be significantly higher in the patient group that had no comorbid disease (p=0.018). Endocrine subscale scores were statistically worse in patients who had psychiatric disease (p=0.057) but the general QoL data were similar with others. It was shown that all QoL scores for all dimensions had statistically significant changes (p<0.001) in terms of hormonal regimes.
Conclusion
The diagnosis of breast cancer was found to be an independent factor that affects social well-being and social life in a negative way. We must give attention to complaints including complaints about sexual life and hormonal status in order to ensure compliance of patients with the required hormonal regimens. By the help of future research, we can improve the prognosis of this disease through increased treatment adherence and belief of patients.
Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in women. In the United States, breast cancer is the most commonly diagnosed and the second most common cause of cancer death in women. In addition, breast cancer is the main cause of death in women aged 40 to 49 years [1,2]. In recent years, the diagnosis and treatment of breast cancer have improved greatly and many patients can expect a long life expectancy. This makes quality of life (QoL) a current issue for breast cancer patients [3]. Despite of the proven activities and acceptable tolerability profiles of hormonal therapy approaches, the adverse effects of hormonal therapy and their negative impact on the QoL are generally underestimated. These adverse effects can change patients' QoL by affecting their physical, functional, emotional and social well-being, and as a result, they can reduce patients' compliance with their cancer treatment [4,5]. Evaluating the effect of cancer treatment and also hormonal therapy upon the QoL in women with breast cancer by using the Functional Assessment of Cancer Treatment (FACT) questionnaire is the main purpose of this trial.
The breast cancer patients treated with adjuvant radiotherapy in Medical School of Ege University between January 2007 and December 2009 were evaluated for this trial after obtaining their informed consent. All of the included patients completed their whole course of treatment for their disease except for the hormonal therapy.
Two hundred and ninety patients completing all their breast cancer treatment in Medical School of Ege University between January 2007 and December 2009 were selected to take part in this trial after obtaining their informed consent. The eligible population number was 297. The first questionnaire was given to the patients explaining the purpose of the study. At the end of the inclusion period, only 122 patients were accepted as suitable for this study. During the application process involving the questionnaires, all of the included patients had neither distant metastasis nor local recurrences. We planned to obtain a minimum of 3 surveys from the included patients. Exclusion criteria were determined as having physiologic disease blocks filling the questionnaires, the patients who could not be obtained control questionnaires, and the patients who don't want to continue (Figure 1). The main reason for being excluded was lack of the required control questionnaires. One hundred patients wanted to be controlled in a different health center.
Functional Assessment of Cancer Treatment-General-version 4 (FACT-G v4) and the endocrine subscale (FACT-ES v4) were used for assessing patients' QoL. FACT-G is designed for assessing all-purpose of QoL in cancer patients and contains 27 questions. The questionnaire consists of 4 subscales which examine physical well-being with 7 items, social well-being with 7 items, emotional well-being with 6 items and functional well-being with 7 items. The FACT-ES subscale querying endocrine symptoms consists of 19 items which assess endocrine complaints and adverse effects. The FACT-G is a 27-item Likert-scaled questionnaire, with response scores ranging from 0 to 4. The response categories include 'not at all', 'a little bit', 'somewhat', 'quite a bit', and 'very much.'
All patients included in this trial were informed about the research and its purposes (IRB approval number: 2007/0014). The validated version in Turkish language of the FACT-G survey and endocrine subscale were used in this trial [6]. The endocrine subscale was used for obtaining information regarding additional concerns of hormonal treatment. The first survey implementation was done after the patient received the whole of their adjuvant cancer treatment except for the hormonal therapy and it was coded as 'basal assessment.' During the basal survey implementation, patients' sociodemographic data, financial status, medical history, comorbid diseases, treatment types used and physical examination results were recorded. The second survey implementation was done 6 to 12 months after the basal surveys. The same issues were recorded except for sociodemographic and financial data. The last survey implementations was done within the last 18 to 24 months of the follow-up period and the recorded data were the same as with the second survey.
Obtained FACT-G and ES questions were evaluated particularly for each included patients according to their standard method.
Data were analyzed using SPSS version 15 (SPSS Inc., Chicago, USA). Paired t-test was used to compare sociodemographic variables, clinical variables and QoL data. The normality test was satisfied, so the one-way analysis of variance (ANOVA) test was used. We had 3 dependent groups (basal, second, and last survey). Because of this, we did not use Wilcoxon Signed Ranks test. We preferred to use the Friedman test for quantitative data and repeated measure one factor analysis based on mean to compare the 3 dependent groups (Table 1). The effect sizes were calculated for each group. Effect sizes up to 0.2 were considered to be small, effect sizes about 0.5 were moderate, and effect sizes of about 0.8 were large. When we used univariate analyses, we found that body mass index, comorbid disease, psychiatric drug usage, job status, surgical procedure type and stage group showed statistically significant difference in terms of QoL. After that we preferred to measure multivariate analyses for these significant factors with general linear model, multivariable analyze technique. In the analyses, p<0.05 was accepted as statistically significant.
We did not prefer to use FACT-B in our surveys. Giving a general QoL with basic, understandable questionnaire was the reason for not using this. That can be given with another research which is interested in a FACT-B survey. The above mentioned issue is the only limitation of this unique trial.
One hundred twenty-two breast cancer patients were included in this research. Whole included patients were followed without any distant metastasis or local recurrences. The sociodemographic data of patients are shown in Table 2. Additionally, the clinical features of the patients are illustrated in Table 3.
The comparable evaluation of the relationship between the basal (before hormonal treatment) QoL survey data and general patient characteristics shows the relationship between independent variables and the basal QoL (Table 4).
When patients were grouped in terms of age, no statistically significant differences were found. But the endocrine subscale scores were higher for the >60 years group than younger ones. The other dimension scores were low in the elderly patient group.
The body mass index (BMI) of patients was grouped by using the criteria of BMI >30 to define obesity. There was a statistically significant relationship between being overweight (BMI >30) and improvement in the social well-being score (p=0.028, multivariable analyses values; p=0.029, R2=0.045:R squared=coefficient of determination, ES=0.57)
In order to evaluate the effect of comorbid disease on QoL, patients were divided into a group with at least one comorbid disease and a group without any comorbid disease. The functional well-being scores were detected to be significantly higher in the patient group that had no comorbid disease (p=0.018, multivariable analyses values; p=0.017, R2=0.040, ES=0.048).
When we compared the basal QoL assessments in terms of drug usage for psychiatric disease, endocrine subscale scores were statistically worse in patients who had psychiatric disease (p=0.057, multivariable analyses values; p=0.063, R2=0.021, Ŋ2: 0.029) but general QoL data were similar to others.
Social well-being scores represented an increasing trend in the nonworking patients group. On the other hand, FACT-G QoL scores were high in educated women (at least elementary school). QoL was affected by financial status, as well. Functional well-being scores were high in patients owning >1,000 Turkish lira monthly income.
It was also seen that QoL was affected by applied surgical procedures used and higher QoL scores were seen in the breast-conserving surgery group (p=0.051, multivariable analyses values; p=0.074, R2=0.019, ES=0.027). In order to evaluate the effect of the stage of breast cancer on QoL, patients were divided into two groups: early stage group and locally advanced stage group. Functional well-being dimension scores were surprisingly higher in the locally advanced group than in the early stage group (p=0.024, multivariable analyses values; p=0.029, R2=0.032, ES=0.040).
The effect of marital status, number of pregnancies, residence in the village or city, hemoglobin levels, and chemotherapy use on QoL could not be seen.
The included patients were grouped according to hormone receptor positivity and were compared in terms of the independent variables before assessing alterations of the QoL dimensions in the follow-up period. The two groups were found to be similar in terms of this comparison. The statistically significant alterations are shown in Table 1 for the hormone receptor (+) group. Endocrine subscale results showed impairment in the first control assessments for hormone receptor (+) patients. The decreased scores had an increasing trend in the second questionnaires but they did not reach basal levels. Our attention was drawn to the fact that the scores of functional and physical well-being increased in the follow-up period. Whole alterations were found to be statistically significant (p<0.001 for all dimensions). When we compared the effect of the sizes of the alterations, endocrine symptoms represented the biggest effect upon QoL (ES=0.706). The effect of endocrine complaints could be seen in the FACT-ES scores as well (ES=0.724).
The situation was different for hormone receptor (-) patients. Their social well-being and endocrine subscale scores had some decrease during the follow-up period. But they did not recover at the end of this trial.
The hormone receptor (+) patient group was divided into two subgroups according to whether hormonal treatment was with tamoxifen or an aromatase inhibitor. The analyses concerning alterations during the follow-up period were assessed in order to evaluate whether any differences were present or not. We chose not to compare the adjuvant tamoxifen group with the adjuvant aromatase inhibitor group. The characteristics of these two groups, in terms of age, menopausal status, and physical conditions were different. The protocol of endocrine treatment changes with the menopausal status of patients so tamoxifen is used for premenopausal women, and, aromatase inhibitors are used for postmenopausal women. The comparison of QoL assessment may not give accurate and pathfinder results.
The alterations of QoL scores for the patients who received adjuvant tamoxifen treatment during the follow-up period are shown in Table 1. It was seen that all QoL scores for all dimensions had statistically significant changes (p<0.001). All QoL scores were decreased in the first control questionnaires obtained 3 to 6 months after tamoxifen usage except for the functional well-being dimension. These decreased scores showed an increasing trend in the second control questionnaires except for the emotional well-being dimension scores which continued to show decreasing trend.
Alterations of QoL scores for patients who used aromatase inhibitor are given in Table 1. Physical, emotional, and social well-being dimension scores showed impairment in the first control questionnaires but these impaired scores started to recover in the second control questionnaires. In addition to this, functional well-being dimension scores showed an increasing trend during the follow-up. But the endocrine subscale scores and accordingly the FACT-ES scores had approximately a 50% decrease when compared with the first control questionnaires and these decreased scores were seen to be covered in second control questionnaires. All these data were found to be statistically significant. The most obvious impairment was seen in the endocrine subscale dimensions (p<0.001). The alterations regarding hormonal treatment types are illustrated in Figure 2.
Age is the most important factor expecting to affect QoL. But there is not any research evaluating the direct effect of age on QoL in the literature. de Haes et al. [7] investigated the QoL data of ≥ 70 years aged breast cancer patients including the European Organisation for Research and Treatment of Cancer (EORTC) 10850 randomized study. They performed a survey examining 9 different dimensions of QoL with 36 questions given to the patients. There were not any survival difference between the patients who participated in the QoL subgroup analyses and the surgical procedure (breast-conserving surgery versus total mastectomy) consisting the purpose of trial in terms of QoL. Crivellari et al. [8] reported the reanalyzed data obtained from the trial that studied the effect of chemotherapy on QoL and called it the International Breast Cancer Study Group Trial VII in order to examine the effect of age on QoL. That subgroup analysis showed that adverse effects were seen more commonly in elderly patients than in younger ones, but there could not be detected any significant difference in terms of the QoL scores regarding physical well-being and general dimension of mood between the two groups. The commentaries of the subgroup analysis were done as the elderly patients tended to tell their complaints less than the younger ones [8]. In the evaluation regarding agedependent basal QoL dimension scores of the 122 patients included in our trial, we could not find any statistical significance similar with the literature but elderly patients (>60 age) had high scores regarding the endocrine subscale and low scores for all the other dimensions.
Penttinen et al. [9] performed EORTC The Quality of Life Questionnaire (QLQ)-C30, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) F, Beck's Depression Scale, and The Women's Health Questionnaire (WHQ) (for vasomotor symptoms) on 537 breast cancer patients completing adjuvant treatment in Finland in order to assess QoL. When looking at the surgical approach, 51.6% patients included in the study had mastectomy, 25% had sentinel lymph node dissection and 91.7% patient had early stage breast cancer. Adjuvant treatments were chemotherapy for 91.9% of patients, radiotherapy for 78.4% of patients and hormonal treatment for 82.9% of patients. Sociodemographic data including BMI, comorbid disease, living with a relative and patient-reported physiologic disease affected the scores of the EORTC QLQ-C30 QoL dimensions in that trial. Increased BMI (p<0.001), having comorbid disease (p<0.001), and patient-reported depression (p<0.001) adversely affected QoL, on the other hand, living with a relative had a positive effect on QoL (p=0.045) [8,9]. It was seen in our study that having at least one comorbid disease and being diagnosed with a physiologic disease had a negative effect on QoL. In contrast to the literature, the patients in our study, who had a BMI >30, had improved social well-being scores.
Dorval et al. [10] evaluated the effect of the surgical procedure upon QoL in 124 breast cancer survivor after a postoperative mean 8.8 years of follow-up at 1998. They performed a Psychiatric Symptom Index survey focusing on psychiatric stress experienced by the patients in their study (n=47 for partial mastectomy and n=77 for total mastectomy). They could not find any statistically significant relationship between surgical procedure and QoL scores. Janni et al. [11] investigated the effect of breast-conserving surgery and total mastectomy on QoL by using the EORTC QLQ-C30 questionnaire at 46 months after surgery. An additional 7 questions were asked to patients in order to get information about the applied surgery. It was found that there was not any statistical difference between the two groups in terms of the EORTC QLQ-C30 questionnaire dimensions. However, it was found that mastectomy had a worse cosmetic outcome (p<0.001), caused bigger stress in social well-being (p<0.001) and made a major difference in the outlook of patients (p<0.001) than breast-conserving surgery with the additional questions. The first randomized-controlled research concerning the axillary surgical approach was performed by Veronesi et al. [12]. There were 100 patients for both arms in that trial. Pain intensity, the presence of parasthesia, shoulder motion range and axillary scar appearance were asked about 6 and 24 months after surgery. Although it could not be shown by statistical test results, higher QoL scores were obtained for the group performed sentinel lymph node biopsy alone in the patient specific assessments. The authors of the trial reviewed the literature [13] in order to discuss the effect of surgical procedure on QoL, and claimed that more standard and more recent research is required because of the insufficient number of randomized-controlled studies and the use of nonstandard questionnaires in previous studies. In our study, only the functional dimension of the FACT-G scores was found to be statistically significant high in the partial surgery group. In addition to this, all dimension scores tended to be higher for the partial surgery group than for the total mastectomy group.
The effect of anastrozole and tamoxifen upon QoL was investigated in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial subgroup analyses. FACT-B+ES QoL questionnaires were used for the evaluation [14-16]. It was found that both anastrozole and tamoxifen had similar basal QoL data in those analyses. No statistically significant differences were found despite their different adverse effect profiles. Endocrine symptom scores were impaired after 3 months of usage for both group however they recovered at that point. But they could not reach basal levels. Vaginal dryness/disparony (p=0.022) and loss of libido (p=0.001) were statistically significantly high in the aromatase inhibitor group. The Intergroup Exemestane Study (IES) [17] evaluated the efficiency and reliability of exemestane by using the FACT-B questionnaire in subgroup assessments. The questionnaires were given before and 24 months after tamoxifen and exemestane were given. The difference of effect regarding QoL between the two drugs and the two surveys were assessed. No significant difference was found for the tamoxifen or exemestane arm between the basal and 24th months' QoL scores. In any case, the endocrine subscale scores improved for both arms in the follow-up periods. The assessment made in terms of sexual complaints could not show any statistical significance for both arms, as well. The MA.17 randomized trial [18], assessing letrozole treatment after 5 years of tamoxifen usage with a placebo control, compared the QoL data in the letrozole group (n=1,813) with the placebo group (n=1,799). The Short Form 36-Item Health Survey (SF-36) and the Menopause Specific Quality of Life (MENQOL) were used as a QoL assessment scale. When comparing the letrozole group with the placebo group after 1 or 2 years of treatment period, a small but statistically significant impairment was seen in physical function, body pain, sexual function and stamina dimensions. Nevertheless, no statistical difference in terms of the general QoL score were found between the two groups [18]. The QoL scores of 103 patients treated with hormonal therapy had statistically significant difference between the basal questionnaires and follow-up questionnaires in our research (p=0.001). When we looked at the changes separately; physical and functional well-being dimensions showed an increase in the follow-up surveys, but on the other hand, social well-being, emotional well-being, FACT-G, endocrine subscale and FACT-ES dimension scores showed a significant decrease in the first surveys but they recovered in the second control surveys. The improvements seen in the second surveys could be considered a result of patients getting used to the adverse effects of hormonal treatment, interview with their doctor who gave them exact information for their optimal tolerance and the symptomatic treatment for their complaints that were given by the doctor in controls. Our results were similar with the results in the literature and also with the ATAC trial subgroup analyses in terms of improvements in the second controls which however did not reach basal levels. It was detected that the QoL assessments for 19 patients who didn't have hormonal treatment, comprising the control group in our trial, showed a statistically significant decrease in terms of social well-being and endocrine subscale dimensions during the follow-up period. The significant decrease of social well-being dimension in the first control survey triggered obtained statistically difference (p=0.023). This could be interpreted as an independent effect of the diagnosis of breast cancer on social well-being and also on social life. The decrease in the endocrine subscale dimension according to endocrine complaints was derived from menopausal hormonal status seen after chemotherapy administrations for all hormonal treatment negative (-) patients included in control groups. Improvement was seen during the follow-up period and we expected that there would be fully recovered with more advanced controls. All these discussed data concerning the hormonal treatment (negative) group were compatible with the assessment of the hormonal treatment (negative) arm in the research reported by Fallowfield et al. [19]. The statistically significant differences in the first control were seen in the endocrine subscale and FACT-ES dimensions within the comparison of hormonal treatment groups regarding tamoxifen versus aromatase inhibitor (p=0.001). For the tamoxifen group, the endocrine subscale and FACT-ES scores were found higher than for the aromatase inhibitor group in the first evaluation, but these high scores showed differences in the second evaluation. While the decrease in the endocrine subscale dimension was seen in the tamoxifen group, the same scores increased in the aromatase inhibitor group during follow-up evaluations (p=0.003). It was thought that the complaints depending on endocrine system were become clear by using tamoxifen hormonal treatment. The effect of endocrine complaints on QoL was smaller in women treated with aromatase inhibitors. It caused from their present postmenopausal hormonal status. Hormonal treatment which form an integral part of breast cancer treatment, can be used confidently by taking into account their different adverse effect profiles. Our study has shown that different hormonal treatments have different effects upon QoL. All the QoL dimensions are affected by hormonal treatment separately. However, social well-being is decreased after the diagnosis of breast cancer. The diagnosis of breast cancer is seen as an independent factor affecting social well-being and social life in a negative way. Hormone suppressing treatments, like tamoxifen, can cause significant differences in breast cancer patients' routine life. But we must pay attention to complaints including complaints about sexual life and hormonal status in order to ensure compliance of patients with their required hormonal regimens. On the other hand, we need multi-institutional, researche with large sample size to evaluate with certainty the effects of hormonal treatment on QoL. With the help of future research, we can improve the prognosis of this disease through increased treatment adherence and increased belief of patients in the value of their treatment.
Figures and Tables
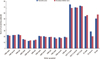 | Figure 2The survey subscale alterations during follow-up period for tamoxifen and aromatase inhibitors users.
PWB=physical well-being; SWB=social well-being; EWB=emotional well-being; FWB=functional well-being; FACT-G=Functional Assessment of Cancer Treatment-General; FACT-G Score=PWB+SWB+EWB+FWB; ES=endocrine subscale.
|
Table 1
The alterations regarding questionnaires' dimensions for hormonal treatment (+) patients, adjuvant tamoxifen users and adjuvant aromatase inhibitor users

FACT=Functional Assessment of Cancer Treatment; AI=aromatase inhibitor; SD=standard deviation; ES=effect size; PWB=physical well-being; SWB=social well-being; EWB=emotional well-being; FWB=functional well-being; FACT-G=Functional Assessment of Cancer Treatment-General; FACT-G Score=PWB+SWB+EWB+FWB; ES=endocrine subscale; FACT-ES score=FACT-G score+ES.
*Analysis of variance in repeated measures.
References
1. American Cancer Society. Breast Cancer Facts & Figures 2007-2008. Atlanta: American Cancer Society;2008. p. 2–8.
2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56:106–130.

3. Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Théberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009). J Natl Cancer Inst. 2011; 103:178–231.

4. Sloan JA, Cella D, Frost M, Guyatt GH, Sprangers M, Symonds T, et al. Assessing clinical significance in measuring oncology patient quality of life: introduction to the symposium, content overview, and definition of terms. Mayo Clin Proc. 2002; 77:367–370.

5. van der Steeg AF, De Vries J, Roukema JA. Quality of life and health status in breast carcinoma. Eur J Surg Oncol. 2004; 30:1051–1057.

6. Functional Assessment of Chronic Illness Therapy Questionnaires-v.4. Functional Assessment of Chronic Illness Therapy;2007. November 16th, 2007. http://www.facit.org/FACITOrg/Questionnaires.
7. de Haes JC, Curran D, Aaronson NK, Fentiman IS. Quality of life in breast cancer patients aged over 70 years, participating in the EORTC 10850 randomised clinical trial. Eur J Cancer. 2003; 39:945–951.

8. Crivellari D, Bonetti M, Castiglione-Gertsch M, Gelber RD, Rudenstam CM, Thürlimann B, et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000; 18:1412–1422.

9. Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, Blomqvist C, Huovinen R, Kautiainen H, et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psychooncology. 2011; 20:1211–1220.

10. Dorval M, Maunsell E, Deschênes L, Brisson J. Type of mastectomy and quality of life for long term breast carcinoma survivors. Cancer. 1998; 83:2130–2138.

11. Janni W, Rjosk D, Dimpfl TH, Haertl K, Strobl B, Hepp F, et al. Quality of life influenced by primary surgical treatment for stage I-III breast cancer-long-term follow-up of a matched-pair analysis. Ann Surg Oncol. 2001; 8:542–548.

12. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003; 349:546–553.

13. Ohsumi S, Shimozuma K, Kuroi K, Ono M, Imai H. Quality of life of breast cancer patients and types of surgery for breast cancer: current status and unresolved issues. Breast Cancer. 2007; 14:66–73.

14. Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol. 2004; 22:4261–4271.

15. Cella D, Fallowfield L. Five-year quality of life (QOL) follow-up of adjuvant endocrine therapy for postmenopausal women in the arimidex (A), tamoxifen (T), alone or in combination (ATAC) trial. J Clin Oncol. 2005; 23:16 Suppl. 577.

16. Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A, et al. Quality of life of postmenopausal women in the ATAC ("Arimidex", tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006; 100:273–284.

17. Fallowfield LJ, Bliss JM, Porter LS, Price MH, Snowdon CF, Jones SE, et al. Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol. 2006; 24:910–917.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download