Abstract
Purpose
The aim of this study was to assess the expression of metabolism-related proteins including glucose transporter 1 (Glut-1), carbonic anhydrase IX (CAIX) and monocarboxylate transporter 4 (MCT4) in breast mucinous carcinoma and to evaluate the implications of the results.
Methods
Immunohistochemical staining for Glut-1, CAIX, and MCT4 was performed on tissue sections from 59 cases of mucinous carcinoma to evaluate the association between the expression of metabolism-related proteins and clinicopathologic factors. Mucinous carcinoma was subclassified into type A and type B according to histopathological characteristics.
Results
Of the 59 patients, 35 patients (59.3%) were type A mucinous carcinoma and 24 patients (40.7%) were type B mucinous carcinoma. Stromal expression of MCT4 was significantly associated with a high histologic grade (p=0.022) and type B mucinous carcinoma (p=0.016). There were significant positive correlations between the expression of Glut-1, CAIX and tumoral expression of MCT4 (p<0.05).
The metabolism of tumor cells is different from that of normal cells in that tumor cells obtain energy from aerobic glycolysis instead of the tricarboxylic acid cycle (TCA cycle). This alternative form of metabolism is referred to as Warburg effect [1]. Because of inadequate oxygen supply due to limited blood flow, rapidly proliferating tumor cells encounter an oxygen-deficient environment. As an adaptive response to this condition, the tumor cells use aerobic glycolysis, which consumes less oxygen than the TCA cycle [2]. The proteins involved in aerobic glycolysis include glucose transporter 1 (Glut-1), an important channel mediating glucose influx into the tumor cell; carbonic anhydrase IX (CAIX), a pH regulating enzyme that is up-regulated in association with glycolysis-related acidosis; and monocarboxylate transporter 4 (MCT4), a transporter mediating extracellular efflux of lactate produced during aerobic glycolysis [3,4]. Expression of these metabolism-related proteins is associated with clinicopathologic parameters in many tumors. Overexpression of Glut-1 was reported to be associated with high-grade and poorly differentiated tumors, invasiveness and distant metastasis [5-7]. In addition, overexpression of CAIX was reported to be associated with high-grade tumors and poor prognosis [8,9]. It can therefore be inferred that the higher the metabolic rate of a tumor has, the more aggressive behavior it shows.
Mucinous carcinoma is a rare variant of invasive breast cancer, which constitutes about 2% of all breast cancers and is characterized by abundant mucin production. Carcinomas that are comprised of at least 90% mucus are defined as mucinous carcinoma [10]. Mucinous carcinoma usually occurs in postmenopausal women and is associated with a lower incidence of nodal metastasis, high expression of estrogen receptor (ER) and progesterone receptor (PR). It is known to have a favorable prognosis [11,12]. Some authors have suggested that mucinous carcinoma be classified into two groups according to the histological characteristics. They are type A (paucicellular), the classical variant with a large amount of extracellular mucin and type B (hypercellular), the hypercellular variant with less mucin and often neuroendocrine differentiation [13].
While expression of metabolism-related proteins in typical breast carcinoma has been previously studied, the expression of these proteins in mucinous carcinoma, a rare variant of breast carcinoma, has not yet been studied. The aim of this study was to assess the expression of metabolism-related proteins including Glut-1, CAIX and MCT4 in breast mucinous carcinoma and to evaluate the implications of the results.
A total of 59 patients who were diagnosed with mucinous carcinoma and underwent surgical excision at Severance Hospital from January 1996 to December 2009 were included in this study. Cases of mixed mucinous carcinoma and invasive ductal carcinoma were excluded. Mucinous carcinoma was diagnosed only when at least 90% tumor produce extracellular mucin [10]. This study was approved by the Institutional Review Board of Yonsei University Severance Hospital. All cases included in the study were retrospectively reviewed independently by experienced breast pathologists (JS Koo and WH Jung). Histologic parameters were evaluated from hematoxylin and eosin (H&E)-stained slides. The histological grade was assessed using the Nottingham grading system [14], and nuclear grade was evaluated according to the modified Black's nuclear grade (1=low grade, 2=intermediate grade, and 3=high grade). Mucinous carcinoma was classified into type A and B according to the following criteria described by Capella et al. [13]: type A (paucicellular), the classical variant with a large amount of extracellular mucin; and type B (hypercellular), hypercellular variant with less mucin and often neuroendocrine differentiation. Clinicopathologic parameters evaluated in each tumor included patient age at initial diagnosis, sex, lymph node metastasis, tumor recurrence, distant metastasis and patient survival.
The materials are human breast cancer tissue samples, which are products of surgical treatment. In addition, our study contains no private information on patients. Therefore, our study has no problems in causing any ethical issue or encroachment of human rights (IRB approval number: 4-2012-0606).
On H&E-stained tumor slides, a representative area was selected and a corresponding spot was marked on the surface of the paraffin block. Using a biopsy needle, the selected area was punched out and a 3-mm tissue core was placed into a 6×5 recipient block. Tissue from the invasive tumor was extracted. More than two tissue cores were extracted to minimize extraction bias. Each tissue core was assigned a unique tissue microarray location number that was linked to a database containing other clinicopathologic data.
All immunohistochemical staining was performed on the formalin-fixed, paraffin-embedded tissue sections. Briefly, 5-µm-thick sections were obtained with a microtome, transferred onto adhesive slides, and dried at 62℃ for 30 minutes. After incubation with primary antibodies for Glut-1 (SPM498, 1:200; Abcam, Cambridge, UK), CAIX (polyclonal, 1:100; Abcam), and MCT4 (polyclonal, 1:100; Santa Cruz Biotechnology, Santa Cruz, USA), immunodetection was performed with biotinylated anti-mouse immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit (DAKO, Carpinteria, USA) with 3,3'-diaminobenzidine chromogen as the substrate. The primary antibody incubation step was omitted in the negative control. Slides were counterstained with Harris hematoxylin.
All immunohistochemical markers were assessed by light microscopy. Pathologic parameters such as ER, PR, and HER2 status were obtained from patients' pathology reports. A cut-off value of 1% or more of positively stained nuclei was used to define ER and PR positivity [15]. HER2 staining was analyzed according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines using the following categories: 0=no immunostaining; 1+ =weak, incomplete membranous staining, less than 10% of tumor cells; 2+ =complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+ =uniform intense membranous staining in at least 30% of tumor cells. HER2 immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases graded as 0 to 1+ were regarded as negative [16]. The cases showing 2+ HER2 expression were evaluated for HER2 amplification by fluorescent in situ hybridization (FISH).
Before FISH analysis, invasive tumors were examined on H&E-stained slides. FISH was subsequently performed on the tested tumor using a PathVysion HER2 DNA Probe Kit (Vysis, Downers Grove, USA) according to the manufacturer's instructions. The HER2 gene copy number on the slides was evaluated using an epifluorescence microscope (Olympus, Tokyo, Japan). At least 60 tumor cell nuclei in three separate regions were investigated for HER2 and chromosome 17 signals. HER2 gene amplification was determined according to the ASCO/CAP guidelines [16]. An absolute HER2 gene copy number lower than 4 or a HER2 gene/chromosome 17 copy number ratio (HER2/Chr17 ratio) less than 1.8 was considered HER2-negative. An absolute HER2 copy number between 4 and 6 or a HER2/Chr17 ratio between 1.8 and 2.2 was considered HER2 equivocal. An absolute HER2 copy number greater than 6 or a HER2/Chr17 ratio higher than 2.2 was considered HER2-positive.
Data were processed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, USA). Student's t-test and Fisher's exact test were used to examine any differences in continuous and categorical variables, respectively. Pearson's correlation analysis was performed to assess the relationships among Glut-1, CAIX, and MCT4 expression. Significance was assumed when p<0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to tumor metastasis and survival. Multivariate regression analysis was performed using the Cox proportional hazards model.
The clinicopathologic characteristics of the patients in this study are shown in Table 1. Among the 59 patients, 35 (59.3%) had type A mucinous carcinoma and 24 (40.7%) had type B mucinous carcinoma. There was no difference in clinicopathologic parameters between the two groups, except that type B mucinous carcinoma had a higher histological grade than type A (p=0.040). Tumor recurrence occurred in only one patient (1.7%) and no patient died of breast cancer.
The correlation between the expression of metabolism-related proteins and clinicopathologic factors is shown in Table 2 and Figure 1. Stromal expression of MCT4 was significantly associated with high histologic grade (p=0.022) and type B mucinous carcinoma (p=0.016). Although not significant, CAIX expression tended to be associated with type B mucinous carcinoma (p=0.109) and Glut-1 and MCT-4 expression tended to be associated with lymph node metastasis (p=0.129 and 0.109, respectively).
There were significant positive correlations among Glut-1, CAIX and tumoral MCT4 expression (p<0.05). However, there was no correlation between stromal MCT4 expression and other parameters (Table 3).
In this study, we assessed the expression of metabolism-related proteins including Glut-1, CAIX and MCT4 in mucinous carcinoma of the breast and evaluated the implications of the results. This study demonstrated that Glut-1 was expressed in 27% of mucinous carcinoma tissues. Previous studies reported that Glut-1 was expressed in 40% to 50% of invasive ductal carcinoma (IDC), not otherwise specified (NOS) [5,9,17]. In comparison with previous studies, our results show that Glut-1 expression is lower in mucinous carcinoma tissues than in IDC, NOS. Previous studies reported that Glut-1 expression is associated with high histologic grade, high proliferative activity, poor differentiation, ER negativity, PR negativity and a basal-like subtype [9,18,19]. Mucinous carcinoma is known to have low histologic grade, low proliferative activity, high ER positivity and PR positivity. Therefore, it can be expected that mucinous carcinoma tissues would show low expression of Glut-1, and our data confirmed this hypothesis. It can therefore be inferred that mucinous carcinoma has a low rate of aerobic glycolysis.
This study demonstrated that 34% of mucinous carcinoma tissues express CAIX. In comparison with previous studies which reported that 13% to 18% of IDC, NOS tissues express CAIX, we found that CAIX expression in mucinous carcinoma tissues is higher than in IDC, NOS [8,9,18,20,21]. It was reported that CAIX expression in breast cancer is associated with high histologic grade, ER negativity, PR negativity, and basal-like breast cancer [8,9,18,20,21]. From these reports it can be expected that CAIX expression would be low in mucinous carcinoma tissues. However, contrary to expectations, CAIX expression was higher in mucinous carcinoma tissues than in IDC, NOS. However, this comparison is between different studies that used different study groups and research methodologies, and thus it is not statistically significant.
The higher expression of CAIX in mucinous carcinoma compared to IDC can be attributed to histological features. Mucinous carcinoma tissues have a characteristic histology where nests of cancer cells are floating on the mucin pool, consequently the cancer cells are separated from the stroma [11]. For this reason, there is a high probability that tumor cells are far away from the blood vessels and are subjected to tissue hypoxia. Because a previous study reported that CAIX is an indicator of tissue hypoxia [22], CAIX is expected to be highly expressed in mucinous carcinoma tissues.
This study demonstrated that stromal expression of MCT4 is significantly associated with high histologic grade (p=0.022) and type B mucinous carcinoma (p=0.016). MCT4 is expressed in glycolytic cells and mediates extracellular efflux of lactic acid produced during glycolysis [23]. Therefore, there are close correlations between Glut-1, which mediates glucose influx during aerobic glycolysis, CAIX, which is up-regulated in association with glycolysis-related acidosis, and MCT-4, which mediates efflux of lactate produced during aerobic glycolysis. This study showed that there are significant positive correlations between the expression of Glut-1, CAIX and tumoral expression of MCT4 (p<0.05). However, there was no correlation between stromal MCT4 expression and other parameters.
Most previous studies have focused on the tumoral expression of MCT4. However, this study showed that stromal expression of MCT4 was associated with pathological factors. A previous study reported that MCT4 is an oxidative stress indicator of cancer-associated fibroblasts in breast cancer [24]. Therefore, MCT4 is expressed in the stroma of the tumor, and high stromal expression of MCT4 is associated with decreased overall survival [25]. Stromal expression of MCT4 in breast cancer represents the reverse Warburg effect, which refers to the fact that peritumoral stromal cells undergo aerobic glycolysis, thereby producing lactic acid, which is released from the stromal cells with the help of MCT4 and subsequently used as a substrate for oxidative phosphorylation by adjacent cancer cells [24].
However, stromal expression of Glut-1 and CAIX was not observed in this study. Therefore, it is inappropriate to regard the stromal expression of MCT4 in mucinous carcinoma tissues as the reverse Warburg effect. Furthermore, prognostic analysis was impossible due to the excellent prognosis of mucinous carcinoma (tumor recurrence in only one patient [1.7%] and no deaths from breast cancer).
Stromal expression of MCT4 was higher in type B mucinous carcinoma than in type A. There are histological differences between type A and B mucinous carcinoma; type A is a paucicellular tumor with an abundant mucin pool and a solid, trabecular growth pattern, while type B is a hypercellular tumor with neuroendocrine differentiation [13]. In addition to histological differences, type A and B mucinous carcinoma were reported to show different patterns of MUC protein expression. Type A showed luminal/apical expression of MUC1 and MUC2, while type B showed membrano-cytoplasmic expression of MUC1 and MUC2. Type A was negative for MUC5B, while type B showed membrano-cytoplasmic expression of MUC5B [26]. Such differences in the intrinsic characteristics of type A and B mucinous carcinoma may contribute to the difference in tumor microenvironments, which would create distinct differences in MCT4 stromal expression. Further studies of the difference in tumor microenvironments between type A and B mucinous carcinoma are needed.
In this study, we investigated the expression of metabolism-related proteins including Glut-1, CAIX, and MCT4 in breast mucinous carcinoma. In conclusion, stromal expression of MCT4 was higher in type B mucinous carcinoma than in type A, which reflected a difference in the tumor microenvironment.
Figures and Tables
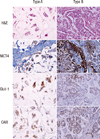 | Figure 1Immunohistochemical staining of monocarboxylate transporter 4 (MCT4), glucose transporter 1 (Glut-1), and carbonic anhydrase IX (CAIX) according to the subtype of mucinous carcinoma. Type B mucinous carcinoma shows high expression of MCT4 in the tumor stroma, but type A mucinous carcinoma demonstrates no expression of MCT4 in the tumor stroma.
MCT4=monocarboxylate transporter 4; Glut-1=glucose transporter 1; CAIX=carbonic anhydrase IX.
|
Notes
References
2. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004; 4:891–899.

3. Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004; 64:6160–6165.

4. Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000; 275:21797–21800.

5. Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993; 72:2979–2985.

6. Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ. Role for glucose transporter 1 protein in human breast cancer. Pathol Oncol Res. 1998; 4:115–120.

7. Stackhouse BL, Williams H, Berry P, Russell G, Thompson P, Winter JL, et al. Measurement of glut-1 expression using tissue microarrays to determine a race specific prognostic marker for breast cancer. Breast Cancer Res Treat. 2005; 93:247–253.

8. Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001; 19:3660–3668.

9. Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011; 26:1279–1286.
10. Tan PH, Tse GM, Bay BH. Mucinous breast lesions: diagnostic challenges. J Clin Pathol. 2008; 61:11–19.

11. Norris HJ, Taylor HB. Prognosis of mucinous (gelatinous) carcinoma of the breast. Cancer. 1965; 18:879–885.

12. Rasmussen BB, Rose C, Christensen IB. Prognostic factors in primary mucinous breast carcinoma. Am J Clin Pathol. 1987; 87:155–160.

13. Capella C, Eusebi V, Mann B, Azzopardi JG. Endocrine differentiation in mucoid carcinoma of the breast. Histopathology. 1980; 4:613–630.

14. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.

15. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.

16. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145.

17. Ravazoula P, Batistatou A, Aletra C, Ladopoulos J, Kourounis G, Tzigounis B. Immunohistochemical expression of glucose transporter Glut1 and cyclin D1 in breast carcinomas with negative lymph nodes. Eur J Gynaecol Oncol. 2003; 24:544–546.
18. Chen CL, Chu JS, Su WC, Huang SC, Lee WY. Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch. 2010; 457:53–61.

19. Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ, Hong SR, et al. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002; 93:1123–1128.

20. Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009; 100:405–411.

21. Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouysségur J, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer. 2007; 120:1451–1458.

22. Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001; 61:6394–6399.
23. Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994; 76:865–873.

24. Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011; 10:1772–1783.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download