This article has been
cited by other articles in ScienceCentral.
Abstract
Approximately 5% of breast cancer patients develop leptomeningeal metastases over the course of their disease. Though several treatments options are available for these patients, their prognosis is typically considered to be poor. We report a case of leptomeningeal failure after a patient underwent prior radiotherapy, radiosurgery, surgery, chemotherapy, and biologic therapy. This patient experienced a prolonged response after receiving bevacizumab and capecitabine. The literature currently contains several reports regarding the use of systemic therapy to manage leptomeningeal metastases from breast cancer, which we summarize. Finally, we review the relevant effects of the patient's treatment modalities and provide a rationale for the mechanism that led to her prolonged response.
Keywords: Bevacizumab, Breast neoplasms, Capecitabine, Meningeal neoplasms
INTRODUCTION
Central nervous system (CNS) metastases are a significant cause of morbidity and mortality in breast cancer patients. Up to 30% of patients diagnosed with breast cancer develop CNS metastases by the end of their disease process as reported by Tsukada et al. [
1]. Of all patients with breast cancer CNS metastases, approximately 20% ultimately display leptomeningeal disease, while patients with estrogen receptor (ER) negative breast cancer may be more likely to develop leptomeningeal disease than those patients with ER positive disease as shown by de la Monte et al. [
2].
Traditionally, leptomeningeal metastases are viewed as occurring late in the natural history of breast cancer, so treatment of these patients is often palliative. However patients with limited metastatic disease are increasingly being treated with the goal of long-term survival. Modalities of treatment being explored include various combinations of whole brain radiation therapy (WBRT), craniospinal irradiation, and intrathecal and systemic chemotherapy. Patients who fail these first-line regimens face nonstandardized treatment options with unknown outcomes. Accordingly, we report a patient with isolated ER/progesterone receptor (PR) negative, human epidermal growth factor receptor 2 (HER2) positive leptomeningeal breast cancer metastases who failed treatment with combined surgery, radiation and systemic therapy who sustained a prolonged remission after beginning treatment with concurrent capecitabine (Xeloda; Hoffman-La Roche Ltd., Basel, Switzerland) and bevacizumab (Avastin; Genentech/Roche, San Francisco, USA).
CASE REPORT
A 45-year-old premenopausal woman was evaluated for a progressively more painful, enlarging right breast mass for approximately 1 year and was found to have breast cancer in February 2005. The patient received modified radical mastectomy with axillary dissection. The tumor involved a majority of the breast including skin and measured 11.0×8.0×4.5 cm. Histopathological analysis revealed invasive poorly differentiated carcinoma, Nottingham's Grade III of III, with immunohistochemistry revealing the tumor to be ER negative, PR negative, and HER2/neu 3+ by immunohistochemistry. Axillary dissection revealed 12 lymph nodes, all involved with carcinoma. Radionucleotide bone scan was negative for signs of metastatic disease, though computed tomography (CT) scan of the chest, abdomen and pelvis revealed a single pulmonary nodule. This lesion was biopsied and pathology revealed metastatic breast carcinoma. Accordingly, the patient was staged pT4b pN3a pM1.
She began chemotherapy April 2005 consisting of weekly paclitaxel (45 mg/m2), carboplatin (2 mg/mL/min), and trastuzumab (2 mg/kg). She completed paclitaxel and carboplatin on October 2005. Adjuvant radiotherapy was offered because the patient had a small volume single metastatic lesion. She completed chest wall and regional nodal irradiation in December 2005. Re-staging images were repeatedly negative at that time. Trastuzumab was changed to 6 mg/kg every 3 weeks in May 2006.
The patient presented to the Emergency Department on September 2006 (1.5 years postmastectomy) complaining of 3 weeks of headaches over her forehead that awoke her at night. CT scan was consistent with brain metastases in the left inferior cerebellum and right posterior temporal lobe. Follow-up magnetic resonance imaging (MRI) revealed a 2.8×3.0 cm oval mass with homogeneously low signal at left inferior cerebellum and a 1.0×1.0 cm area of increased signal within the posterior right temporal lobe. Systemic re-staging at the time of diagnosis of the brain metastasis was negative. The patient was treated with Gamma Knife Radiosurgery, with 15 Gy delivered to the tumor margin (45% isodose line) of the lesion in the cerebellum and 16 Gy delivered to the tumor margin (48% isodose line) of the temporal lobe lesion. She clinically responded to treatment and headaches resolved.
Follow-up MRI in May 2007 revealed tumor progression at the left inferior cerebellum. She underwent suboccipital craniectomy which achieved a gross total resection of the cerebellar lesion. Follow-up imaging in August 2008 again revealed disease progression within the cerebellar resection cavity. There was also increased size of the right temporal lesion. Soon after, the patient received external beam intensity modulated radiation therapy (IMRT) of 54 Gy in 30 fractions to the right temporal lobe and the fourth ventricle.
Unfortunately, follow-up MRI in September 2008 revealed continued progression of the left cerebellar lobe lesion. Clinically, the patient experienced deteriorating balance and significant ataxia. In December 2008 the patient underwent repeat suboccipital craniectomy and again it was felt that gross total or near gross total resection had been achieved. Follow-up imaging remained negative for several months.
In April 2009, the patient began lapatinib 1,200 mg daily in addition to maintenance trastuzumab 6 mg/kg every 3 weeks. MRI of the brain obtained in May 2009 was concerning for continued progression of the right posterior temporal lobe lesion. Follow-up imaging in July and August 2009 revealed worsening enhancement of both the right posterior temporal lesion as well as development of leptomeningeal enhancement along the resection tract and lining of the 4th ventricle. The patient refused cerebral spinal fluid (CSF) sampling, but MRI of the spine was negative. At this time, the decision was made to begin capecitabine (2,000 mg orally twice a day for 2 weeks with 1 week off) and bevacizumab (15 mg/kg intravenously every 3 weeks). She began receiving this therapy the second week of September 2009 (4.5 years postmastectomy).
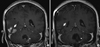
The patient continued being followed with MRI imaging every 4 months. MRI of the brain obtained in November 2009 was significant for interval decrease in the size of enhancing lesions in the right temporal lobe and left inferior cerebellum. Repeated follow-up imaging revealed stable or decreased appearance of both lesions from this time point until September 2011 when she died of complications related to a fall. She remained free of signs of extracranial disease progression 2 years after bevacizumab and capecitabine therapy. During that time, she maintained compliance to the bevacizumab and capecitabine. She had chemotherapy-related anemia prior to initiating this combination, and this did not change appreciably during her treatment. Her blood pressure was consistently measured in the prehypertension range. She developed mild tingling in the hands and feet consistent with hand foot syndrome, but did not require a dose adjustment of capecitabine. Her systemic imaging remained free of disease during the entire time period that she received the bevacizumab and capecitabine. Comparative pretreatment and posttreatment representative images are included in
Figure 1. A summary of the treatment timeline is found in
Figure 2.
DISCUSSION
The current case report depicts a growing problem in the management of patients with metastatic breast cancer: leptomeningeal failure. In a recent surgical series of over 100 patients with resected brain metastases by Jensen et al. [
3], breast cancer was the most common type of cancer to experience leptomeningeal failure with 5 of 15 patients treated with surgery for intracranial metastatic breast cancer ultimately experiencing leptomeningeal failure. The clinical dilemma presented by leptomeningeal disease results from the fact that the blood brain barrier commonly prevents the adequate penetration of systemic chemotherapy. Several strategies exist for treating leptomeningeal metastases including radiotherapy, intrathecal chemotherapy and systemically administered chemotherapy.
Full craniospinal irradiation (CSI) may lead to complete or partial response in approximately half of breast cancer patients with leptomeningeal disease, though it is not curative and reports are limited as shown by Hermann et al. [
4]. Furthermore, CSI alone can be quite toxic, leading to severe cytopenias, nausea, depletion of bone marrow reserve, and an overall worsening of performance status. Because of the toll that CSI can take on patients, it has been used judiciously in the population of cancer patients with leptomeningeal disease, with breast cancer patients having good performance status and minimal disease outside of the CNS as the only candidates. While the current patient was a candidate for CSI, the focal nature of her intracranial leptomeningeal disease and the plan for future chemotherapy made the risks associated with CSI a concern.
More commonly, WBRT can be used to treat multiple leptomeningeal lesions while prophylaxing against occult micrometastases. WBRT is generally much better tolerated than CSI, though it too can lead to neurocognitive decline. Wasserstrom et al. [
5] reported a series of patients with leptomeningeal spread of cancer, of which 46 patients had breast cancer, and 43 underwent WBRT. Among the breast cancer patients, there was a 61% crude rate of stabilization or improvement of symptoms with WBRT. Nearly half of those with an initial response or stabilization ultimately experienced disease relapse within the leptomeninges. In the case of the current patient, prior doses of radiosurgery and local field radiotherapy precluded any further WBRT.
Limited data exist for intrathecal use of hormonal therapy, though one case report by Peroukides et al. [
6] illustrates long-term survival in a patient with leptomeningeal metastases of ER/PR positive, HER2 negative invasive ductal breast adenocarcinoma treated with intrathecal letrozole. A case report by Ferrario et al. [
7] demonstrates the potential of intrathecal trastuzumab with breast cancer CNS metastases with reported survival more than 6 years from time of first dose. Good results have been reported using a variety of dosing schemes, most commonly 20 to 30 mg every week. A case report by Stemmler et al. [
8] using intrathecal trastuzumab and methotrexate with cytarabine suggest a potential role for combined therapy, though further investigation is needed to elucidate the outcome of such treatments. A retrospective analysis by Clatot and colleagues [
9] suggested that single agent intrathecal methotrexate results in cytologic and clinical improvement in about half of patients with breast cancer leptomeningeal metastases. Intrathecal single-agent slow-release cytarabine led to a response rate of 28% in patients with leptomeningeal metastases from breast cancer as reported in a series by Jaeckle et al. [
10]. The major problem with intrathecal chemotherapy is the inability to penetrate several millimeters into bulk disease. Intrathecal therapy can also lead to systemic toxicities.
Systemic therapies may ultimately represent the optimal treatment options for leptomeningeal disease from breast cancer. After several local failures and then leptomeningeal failure at the surgical site in the posterior fossa, the patient in our report ultimately achieved a prolonged response after being treated with bevacizumab and capecitabine.
Figure 1 depicts pretreatment and posttreatment MRI for this regimen.
Table 1 summarizes currently available cases of leptomeningeal breast carcinoma in the literature, including treatment strategies and outcomes, for patients treated with various combinations of capecitabine, trastuzumab, lapatinib, and bevacizumab.
One report by Ekenel et al. [
11] of seven patients treated with capecitabine showed several patients achieving complete response for brain and leptomeningeal metastases from breast cancer. A summary of reported cases in the literature using capecitabine in the treatment of brain or leptomeningeal metastases from breast cancer is located in
Table 1. The current report represents among the most durable responses reported in the scientific literature for a patient receiving capecitabine for breast cancer with CNS disease. We have hypothesized that the use of bevacizumab has aided in the prolongation of this response. Bevacizumab is a humanized monoclonal antibody that binds to and inhibits vascular endothelial growth factor A. While the blood brain barrier is disrupted by metastases, bevacizumab may help to normalize this disrupted tumor vasculature. This normalization decreases edema within the brain, leading to decreased hydrostatic pressure and possible increased penetration of systemically administered agents. With the ability of bevicuzamab to stabilize the tumor vascular endothelium, capecitabine which at baseline displays some ability to penetrate the blood brain barrier was perhaps more able to enter the subarachnoid space. It is unclear as to whether the effect of bevacizumab was synergistic versus additive in the current case because a prolonged response to capecitabine alone has been previously been reported. However, the current case is among the most durable reported in the literature, and the patient did not die directly from tumor progression, but rather from consequences of a fall from neurologic impairment. This report represents the first case for the combination of capecitabine and bevacizumab leading to prolonged treatment response for leptomeningeal breast cancer in the literature. This combination may be worthwhile investigating in a prospective manner.
Although several treatment options are available for breast cancer patients with leptomeningeal metastases with possibility for long-term response, reported outcomes are highly variable and long-term response is uncommon. A well-designed prospective trial would greatly benefit these patients and would potentially allow providers to recommend more efficacious treatment options.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download