Abstract
The skeletal muscle is an unusual site for metastasis from breast cancer. We present two cases of breast cancer that relapsed as skeletal muscle metastasis without other distant organ metastasis. We performed the core needle biopsy of metastatic sites and confirmed discordance in estrogen receptor, progesterone receptors, and human epidermal growth factor receptor 2 expression between primary breast cancer and skeletal muscle metastases. In the second case, we found the skeletal muscle metastasis through F-18 fluorodeoxyglucose positron emission tomography/computed tomography scans (PET/CT). Intramuscular hot spots on PET/CT scans should be considered as a sign of metastasis even in the absence of abnormalities on computed tomography scans. Our patients received systemic chemotherapy, and showed a partial response. Further studies are needed to determine the prognosis and proper management of isolated skeletal muscle metastasis in breast cancer.
Breast cancer is the second most prevalent cancer in women in South Korea. Over the past few decades, significant advances have been made in adjuvant therapy of breast cancer, but 20% to 30% of patients treated with adjuvant therapy after mastectomy, still experience recurrence of cancer for the rest of their life [1]. Recurrence or metastasis can occur in any organ, but metastases to skeletal muscle are rare as the first manifestation of metastasis of breast cancer. We report two cases of breast cancer that metastasized to skeletal muscle after mastectomy.
A 51-year-old woman was referred to Division of Medical Oncology on March 23, 2011, for elevated serum levels of the tumor marker CA 15-3. At that time, she had been taking tamoxifen (20 mg daily) for 6 months following mastectomy in 2009. She had no symptoms and there was no clinical evidence of tumor recurrence on the breast computed tomography (CT) scan, bone scan, and F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) performed in February 2011.
The woman's medical history showed that she had been diagnosed with right breast cancer (cT2N2M0) in January 2009, and underwent modified radical mastectomy after three cycles of neoadjuvant chemotherapy with docetaxel and epirubicin. An histopathological examination showed invasive ductal carcinoma with nuclear grade 3, and 4 metastatic lymph nodes out of the 5 dissected axillary lymph nodes. Immunohistochemistry studies showed positive staining for the estrogen receptor (ER) protein, and negative staining for progesterone receptor (PR) protein and the human epidermal growth factor receptor 2 (HER2) (score 0). She received an additional three cycles of adjuvant chemotherapy with docetaxel and epirubicin until June 2009, and then adjuvant radiotherapy on the right chest wall and regional lymphatics. In November 2009, a 2.3-cm diameter mass was found on surveillance CT of the left breast, and invasive ductal carcinoma was confirmed by core needle biopsy (cT2N2M0). She underwent modified radical mastectomy of her left breast. An histopathological examination revealed invasive ductal carcinoma with nuclear grade 2 and lymphovascular invasion. There were 5 lymph node metastases detected in 9 resected axillary lymph nodes. The expression of ER, PR, and HER2 was concordant with the prior right breast cancer: ER positive, PR negative, and HER2 negative (score 0). She received adjuvant radiotherapy and six cycles of adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) until October 2010.
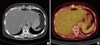
Her serum level of CA 15-3 increased from 24.10 U/mL in October 2010 to 121.94 U/mL (normal range, 0-28 U/mL) in March 2011, when she was presented to our department. Because there was no clinical evidence of tumor recurrence, we planned to carefully follow up tumor markers and imaging tests. Her serum level of CA 15-3 continued to increase to 829.77 U/mL in August 2011, but there was no evidence of tumor recurrence on the chest CT and bone scan, performed in April and July 2011. In October 2011, she complained of intermittent stabbing pain in the epigastric area. On physical examination, there was no palpable mass in her abdomen, but she had mild tenderness with deep palpation in the epigastric region. We performed chest and abdominal CT scans and PET/CT. CT scans showed multiple round or oval masses with the density of soft tissue in the subcutaneous and rectus abdominis muscle of the epigastric region (Figure 1A), and enlargement of lymph nodes in the peripancreatic and para-aortic regions. PET/CT also showed multiple nodules of various sizes with increased FDG uptake (SUVmax, 7.1) in the upper anterior abdominal muscles (Figure 1B), and multiple enlarged lymph nodes with increased FDG uptake (SUVmax, 4.3) in the upper para-aortic region. A core needle biopsy of the abdominal wall mass was performed, and a pathological examination revealed metastatic carcinoma (Figure 2A), that was positive for ER, negative for PR, and positive for HER2 (score 3). Thus, the HER2 status was discordant between the primary breast cancer and the metastatic carcinoma. She received six cycles of trastuzumab and docetaxel chemotherapy followed by three cycles of trastuzumab monotherapy. After the third and sixth cycles of combination chemotherapy, follow-up CT scans showed partial regression of multiple metastatic masses in the subcutaneous and rectus abdominis muscles. However, after an additional three cycles of trastuzumab monotherapy, follow-up CT scans showed an increase in size of the metastatic carcinoma in the subcutaneous and rectus abdominis muscles, and her disease progressed. She therefore started second-line capecitabine and lapatinib combination chemotherapy in June 2012.
A 52-year-old woman visited our hospital with a palpable left neck mass in April 2010. Her medical history showed that she had been diagnosed with left breast cancer (cT2N0M0) in December 2004, and she underwent modified radical mastectomy of the left breast in our hospital. An histopathological examination showed invasive ductal carcinoma with nuclear grade 2, and the absence of axillary lymph node metastases. Immunohistochemistry studies showed positive staining for ER and PR and negative staining for HER2 (score 0). She received five cycles of adjuvant CMF chemotherapy followed by tamoxifen (20 mg daily) for 2 years. She then on her own authority stopped taking tamoxifen and ceased regular surveillance.
We performed CT scans of the neck and breast, bone scan, and PET/CT. CT scans showed multiple enlarged lymph nodes in the left axillary region and the left lower cervical chain, and PET/CT showed multiple conglomerated enlarged lymph nodes with high FDG uptake (SUVmax, 7.02) in the left axillary, retropectoral, subclavian, internal mammary, and cervical lymph nodes. There were no metastatic sites except the lymph nodes. A core needle biopsy of the left axillary lymph node was performed, and pathological examination revealed metastatic adenocarcinoma, which was positive for ER, negative for PR, and equivocal for HER2 (score 2). She was postmenopausal based on the serum follicle-stimulating hormone level, and she started receiving the nonsteroidal aromatase inhibitor letrozole in May 2010. She also received radiotherapy to her left chest wall and regional lymphatics including the cervical chain (total dose, 5,040 cGy) between August 18 and September 30, 2010. Follow-up CT scans showed a complete regression of the previously invaded lymph nodes in November 2010.
About 10 months later, she complained of a mild throbbing pain in the right buttock area that was aggravated by walking. On physical examination, there was no palpable mass and tenderness in the buttock area. CT scans of the abdomen-pelvis and PET/CT were performed in August 2011. Abdomen and pelvis CT scans showed no notable findings, but PET/CT showed multiple new enlarged lymph nodes with increased FDG uptake (SUVmax, 15.4) in the right iliac chain and a nodular lesion of about 2.5 cm in diameter (SUVmax, 20.1) in the right gluteus maximus muscle. We decided to switch from letrozole to the steroidal aromatase inhibitor exemestane on disease progression. Three months later, a follow-up abdominal CT scan showed no discernible change, so the patient continued to take exemestane. After 6 months of taking exemestane, PET/CT showed an increase in size of the right gluteal muscle mass and right iliac lymph nodes (Figure 3A). There was no evidence of local recurrence or other distant metastases. Based on PET/CT images, a poorly defined, mildly enhanced mass of 3 cm in diameter was identified in the right gluteus maximus muscle upon abdominal CT scan (Figure 3B). Therefore, we performed ultrasound-guided core needle biopsy of this gluteal muscle mass in February 2012. Pathological examination revealed invasive ductal carcinoma (Figure 2B), which was negative for ER and PR, and equivocal for HER2 (score 2). HER2 gene amplification was not observed by fluorescence in situ hybridization (HER2/CEP17 ratio, 1.26). Thus, expression of ER showed discordance between the primary breast cancer and the metastatic carcinoma. The patient received palliative chemotherapy with docetaxel and epirubicin. After two cycles of chemotherapy, a follow-up abdominal CT scan showed partial regression of the gluteal muscle mass and iliac lymph nodes, with a decrease in diameter of the gluteal muscle mass from 3.0 to 2.1 cm. She continued to receive chemotherapy and follow-up.
Skeletal muscle metastasis is relatively rare compared with bone metastases. A few cases of radiologically apparent or clinically symptomatic skeletal muscle metastases have been reported in different tumor types [2-4]. It has been suggested that skeletal muscle is relatively resistant to metastatic disease because of its hostile microenvironment. Factors that make skeletal muscle hostile include muscle motion resulting in mechanical tumor destruction, inhospitable muscle pH, the muscle's ability to remove tumor-produced lactic acid associated with angiogenesis, and the activation of lymphocytes and natural killer cells in skeletal muscle [5,6]. However, according to data from a large autopsy series, subclinical metastases to skeletal muscle may be more common than generally thought, and the incidence has been reported to range from 0.2% to 17.5% [5,7]. The underdiagnosis of skeletal muscle metastases in clinical practice may be related to the observation that they are often manifested as part of the disseminated disease and furthermore, in some cases, it is difficult to detect skeletal muscle metastasis with the generally used CT scans.
Skeletal muscle metastasis from breast cancer is also uncommon, and is often manifested as disseminated disease with multiple organ metastasis [8]. Ogiya et al. [8] reported a case of breast cancer with an isolated metastasis into the abdominal wall muscle, with a review of 13 previously reported cases of which four presented as an isolated skeletal muscle metastasis without other distant metastases. The metastatic sites were the paraspinal muscle, scalene muscle, iliopsoas muscle, and extraocular muscle. In our patients, one relapsed with an abdominal wall muscle metastasis without other distant organ metastasis, and the other showed gluteal muscle metastasis with involvement of iliac lymph nodes. We performed a muscle biopsy for proper diagnosis, and a pathological examination revealed diffuse infiltration by cancer cells with disruption of the muscle fascicles.
Recently, several studies have reported a discordant HER2 status between primary and metastatic sites in breast cancer. Niikura et al. [9] reported that the incidence of discordance for ER, PR, and HER2 between primary and metastatic tumors was 18.4%, 40.3%, and 13.6%, respectively. However, to the best of our knowledge, no previous reports examined the hormone receptor or HER2 status of the primary tumor and metastatic skeletal muscle lesions. In our patients, we confirmed discordant ER, PR, and HER2 status between the primary breast cancer and the metastatic skeletal muscle lesions. Therefore, our cases support the need for the biopsy of metastatic skeletal muscle lesions to determine accurate diagnosis and proper management.
Skeletal muscle metastasis generally manifests itself as a painful mass in the involved area; our patients also complained of mild muscular pain [10]. However, skeletal muscle metastasis may be an incidental finding in imaging studies without symptoms [10]. Therefore, more careful monitoring of imaging results for musculoskeletal structures is required when evaluating the response. CT is generally used for staging and response evaluation, and provides information about the extent of the mass in skeletal muscle and its relationship with adjacent structures. However, most of the body's musculature is outside the scanned region of the chest and abdominal CT, and some lesions may be isodense compared with the surrounding muscle, making it difficult to differentiate the metastatic lesion from the surrounding muscle [5,7]. Magnetic resonance imaging (MRI) is the gold standard for imaging muscle disease; it shows features of muscle metastases that are relatively typical, consisting of round or oval intramuscular masses with well-defined margins and marked enhancement [7]. However, MRI is not commonly used in daily practice because it has high cost, long scanning times, and a limited field of view. The number of intramuscular metastases detected has increased since the introduction of PET/CT [11,12]. A recent study, in which unsuspected intramuscular metastases were found by PET/CT in 20 of 39 cases, showed that PET/CT has higher sensitivity than MRI for detecting skeletal muscle metastases [7]. In our second patient, we found a gluteal muscle metastasis by PET/CT that was initially missed on CT. Therefore, PET/CT may be a sensitive tool for detecting skeletal muscle metastases.
The prognosis and appropriate treatment of skeletal muscle metastasis are currently uncertain [8]. Therapeutic options include radiotherapy, chemotherapy, and surgical excision. In previous reports, surgical excision was recommended in selected patients such as those with a painful isolated mass [5]. We treated our patients with chemotherapy and confirmed a partial response and relief of symptoms. Further studies are needed to determine the prognosis and proper diagnostic and therapeutic strategies for skeletal muscle metastasis in breast cancer.
Figures and Tables
Figure 1
(A) Computed tomography scan of the abdomen, showing soft tissue density lesions in rectus abdominis muscle. (B) F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography, showing abdominal muscle mass with increased FDG uptake (SUVmax, 7.1).
References
1. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010. 28:1684–1691.

2. Acinas García O, Fernández FA, Satué EG, Buelta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984. 31:57–67.
3. Camnasio F, Scotti C, Borri A, Fontana F, Fraschini G. Solitary psoas muscle metastasis from renal cell carcinoma. ANZ J Surg. 2010. 80:466–467.

4. Girard M, Kelkel E, Peoch M, Grand S, Massot C. Metastasis in the psoas muscle disclosing breast carcinoma. Ann Med Interne (Paris). 1992. 143:492–494.
5. Molina-Garrido MJ, Guillén-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011. 13:98–101.

6. Doo SW, Kim WB, Kim BK, Yang WJ, Yoon JH, Song YS, et al. Skeletal muscle metastases from urothelial cell carcinoma. Korean J Urol. 2012. 53:63–66.

7. Emmering J, Vogel WV, Stokkel MP. Intramuscular metastases on FDG PET-CT: a review of the literature. Nucl Med Commun. 2012. 33:117–120.
8. Ogiya A, Takahashi K, Sato M, Kubo Y, Nishikawa N, Kikutani M, et al. Metastatic breast carcinoma of the abdominal wall muscle: a case report. Breast Cancer. Epub 2012 Mar 2. DOI: http://dx.doi.org/10.1007/s12282-012-0352-3.

9. Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012. 30:593–599.

10. Tuoheti Y, Okada K, Osanai T, Nishida J, Ehara S, Hashimoto M, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004. 34:210–214.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download